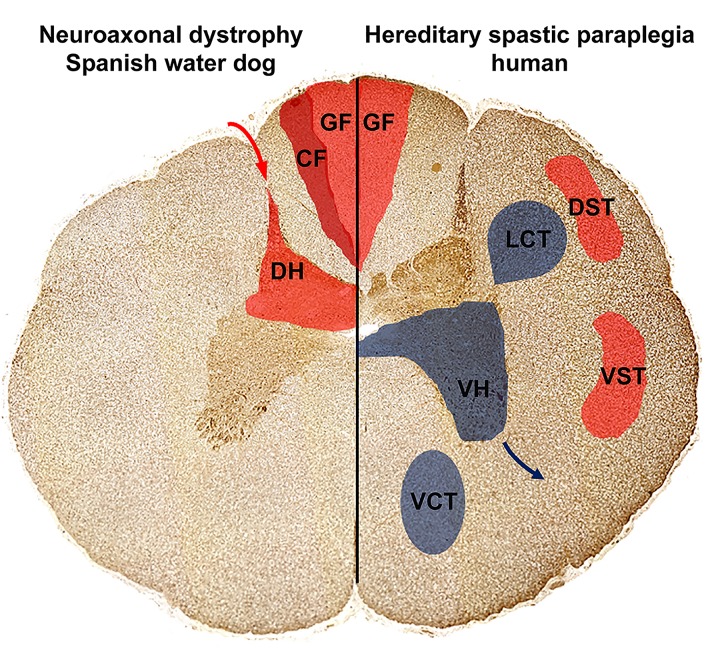
Fig 6. Distribution of cervical and thoracic spinal cord spheroids in Spanish water dogs with NAD compared to mostly affected areas in human hereditary spastic paraparesis (HSP).
In NAD affected Spanish water dogs (left), spheroids and neuronal loss were restricted to the sensory, ascending pathways localized to the grey matter of the spinal cord dorsal horn and single large spheroids were detected within the cuneate and gracile fasciculus. This might explain the clinical signs as gait disturbances, proprioceptive deficits, decreased spinal reflexes and urinary incontinence. In other human forms of NAD, HSP and Pla2g6 knock-out mice (right), spinal cord spheroid formation is accentuated in the sensory pathways including the gracile fasciculus as well as the corticospinal tracts. Furthermore, also the descending motor pathways including the ventral horns as well as the descending pyramidal tracts are affected. Note that spinal cord histology of TECPR2 associated HSP in humans is unknown. Ascending, sensory pathways (red; transmission of sensory signals from the periphery (red arrow) via dorsal horn (DH) towards the brain): Dorsal funiculus composing of gracile fasciculus (GF) and cuneate fasciculus (CF); Spinocerebellar tracts with: dorsal spinocerebellar tract (DST) and ventral spinocerebellar tract (VST); Descending, motor pathways (blue; signal transmission via the ventral horn (VH) neurons towards the muscles; blue arrow): Pyramidal tracts with lateral corticospinal tract (LCT) and ventral corticospinal tract (VCT).