Straight-chain, or normal, alkanes are the simplest class of organic compounds and are prominent components of petroleum, in which they ultimately derive from geo-thermo-chemical reactions acting over millions of years on biological debris (1). Many of the same compounds are also produced selectively by enzymatic reactions in the biosphere. With the notable exception of methane, a catabolic end product for some archaea, biogenic hydrocarbons are typically anabolic products that do not accumulate to high concentration. Nonetheless, the biosphere’s capacity for hydrocarbon production is impressive in terms of both functional diversity and evolutionary span. A range of alkanes and alkenes are produced by plants (2, 3), bacteria (4), and insects (5); archaea produce alkenes (6), and even polycyclic aromatic hydrocarbons are produced in termite colonies (7) and possibly by fungi (8). Their functions in these organisms include water balance, self-defense, signaling, and membrane architecture. Algae have long been of interest because of their potential for biofuel production (9); cyanobacterial production of 15- and 17-carbon alkanes, known since 1971 (10), has recently been shown to proceed via enzymatic decarbonylation of the acyl chains typically used for membrane lipid tails (11).
In PNAS, Lea-Smith et al. (12) assess the n-alkane and alkene production capacity of the globally important cyanobacteria Prochlorococcus and Synechococcus. They conduct a genetic dragnet of 51 sequenced isolates to demonstrate that all retain the genetic capacity for hydrocarbon production. They then survey seven representative strains in laboratory culture in 2-L flasks and quantify hydrocarbon production in each, reporting dry weight percentages of 0.149–0.368% and 0.022–0.138% for three Prochlorococcus and four Synechococcus strains, respectively, inclusive of three compounds, n-pentadecane, n-heptadecane, and some 8-heptadecene. This effort represents the first quantification of hydrocarbon production in Prochlorococcus, a genus estimated to account for ∼15% of net ocean primary productivity (13).
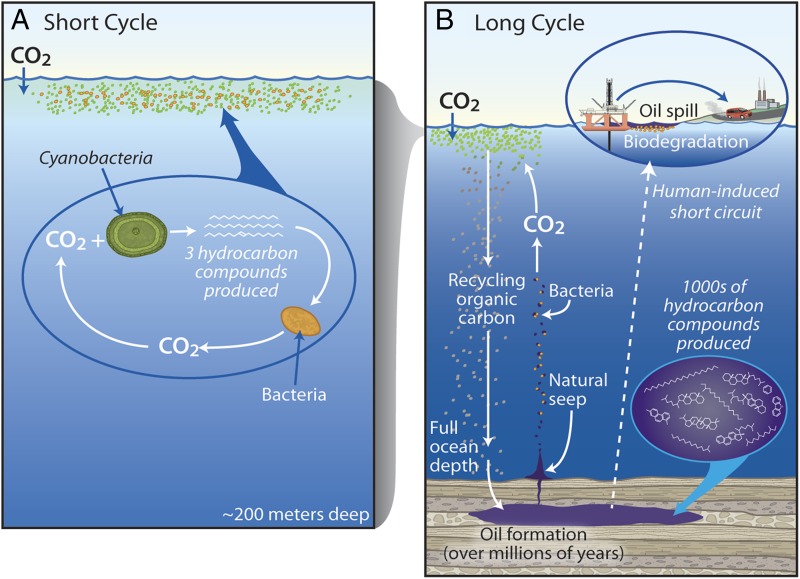
By scaling the fractional yield of alkanes produced in these seven laboratory cultures to the global productivity of both genera, the authors calculate annual global-scale hydrocarbon production of ∼308–771 million tons. These numbers are staggering in the frame of reference of oceanic hydrocarbons, and if alkane productivity truly is of this magnitude, then Prochlorococcus alone may produce as much hydrocarbon as does Saudi Arabia (14). However, evidence to support hydrocarbon production of this magnitude is scant because accumulation has not been observed. Instead, the authors argue based on their scaling of the culture studies that a latent hydrocarbon cycle must exist in the ocean, in which cyanobacterial hydrocarbons feed hydrocarbon-degrading bacteria. They propose that this tight coupling of cyanobacterial production to bacterial biodegradation in the surface ocean represents a “short-term hydrocarbon cycle” that they contrast to the “long-term hydrocarbon cycle” of geo-thermo-chemical production and seepage that is presently being short-circuited by industrial production and use of petroleum (Fig. 1). The authors argue that these cycles are linked by the idling of hydrocarbon-degrading microbes, sustained by cyanobacterial hydrocarbons and ready to degrade releases from natural seeps and anthropogenic oil pollution (15).
Fig. 1.
Cartoon representing the (A) short-term hydrocarbon cycle proposed by Lea-Smith et al. (12) and (B) long-term hydrocarbon cycle. Both cycles predate human activities, derive from algae, and rely on bacterial hydrocarbon consumption, but they differ in where and how the hydrocarbons are produced, time to produce, specificity of the products, and turnover time. By tapping geologic reservoirs humans have short-circuited the long cycle (Inset). Lea-Smith et al. (12) propose that the short-term cycle primes the ocean's microbiome to manage hydrocarbon influx from the long-term cycle.
These latest results highlight a disparate array of questions that we consider in the paragraphs below:
-
•
How do cyanobacterial hydrocarbons partition between evaporation, export to the deep ocean, and surface-ocean accumulation and cycling?
-
•
What would a short-term hydrocarbon cycle imply for intrinsic bioremediation of oil spills?
-
•
For what purpose do cyanobacteria produce hydrocarbons?
-
•
Can cyanobacterial hydrocarbons be harvested as biofuel?
Although the magnitude of cyanobacterial hydrocarbon production in nature remains unsettled, support for its importance can be gained from alkane concentration measurements in the water column (12) and remote marine atmosphere (16, 17). Although the atmospheric concentrations are exceptionally low, they indeed reveal elevated concentrations of n-pentadecane and n-heptadecane relative to other alkanes, with phytoplankton implicated as the source. Interestingly, the elevated concentrations are readily apparent in the tropical Pacific (16), where cyanobacteria account for most of the primary production, but are absent or transient in coastal and North Atlantic waters, where eukaryotic phytoplankton tend to be dominant. Other hydrocarbons, including the branched alkanes pristane and phytane, were of greater abundance in these environments. Although these findings support the occurrence of cyanobacterial hydrocarbon production in the ocean, the detection of these hydrocarbons in the atmosphere demonstrates that evaporation and photochemical loss need to be reconsidered alongside the short-term, or latent, hydrocarbon cycle.
Among other implications, Lea-Smith et al. (12) suggest that cyanobacterial alkanes could serve to sustain a population of hydrocarbon-degrading bacteria over broad ocean expanses, like the idling of a global motor. In this scenario, hydrocarbon degraders are poised to bloom in response to pulsed inputs such as oil spills. Future efforts to explore this far-reaching hypothesis will need to grapple with several major obstacles. First, n-alkanes are only one compound class in oil (Fig. 1), and a population that maintains n-alkane biodegradation pathways need not also maintain the pathways required for degradation of the more complex hydrocarbons that can constitute a large fraction of oil. Further, the full context of the ocean’s hydrocarbon-degrading microbiome requires consideration of all of the inputs (15), including atmospheric, continental, seepage, and other biota. Third, the availability of cyanobacterial hydrocarbons to biodegraders will depend heavily on their physical state: Do they partition with cellular mass, adsorb to particles, form vesicles, or partition to form biological slicks at the sea surface? Fourth, to what extent do evaporation and sedimentation reduce the inputs to the short-term hydrocarbon cycle? For a recent controlled release experiment in the North Sea, 95% of n-pentadecane was found to evaporate within the first day following a surface release (18). Finally, the concentration of oil during an active spill is likely to exceed the concentration of cyanobacterial hydrocarbons by a factor of a million, with dramatic implications for access to substrate and nutrients by the microbial responders. In the case of the Deepwater Horizon disaster, for example, alkanes persisted for long periods (19), underscoring the inconsistency with which oceanic bacteria respond to spills.
Whereas the magnitude and biogeochemical importance of cyanobacterial hydrocarbon production remains an open question, a biological function(s) for alkanes in cyanobacteria is certain but elusive. Supporting a core biological function is the near ubiquity of hydrocarbon production among marine cyanobacteria as well as the conservation of a common, biosynthetic pathway. Their hydrophobicity, inertness, and abundance point to possible functions for hydrocarbons in cellular membranes and or in lipid vesicles. Cellular membranes, especially the thylakoid, are an appealing possibility as hydrocarbons could blend into the lipid bilayers, where they would modulate permeability, flexibility, curvature stress, and fluidity while being impervious to oxidative damage that plagues highly unsaturated membrane lipids (20). Observed hydrocarbon concentrations are equivalent to ∼10% of bilayer-forming membrane lipids, consistent with low-level blending per the DHA principle (20). A role in lipid vesicles is also appealing because those hydrophobic entities are now thought to be abundant in cyanobacteria (21). In both cases, the use of hydrocarbons in membranes provides an intriguing benefit, because their biosynthesis requires no net use of nutrients; a similar adaptive strategy has been reported for anionic, bilayer-forming lipids (22).
Hydrocarbons still serve as the predominant fuel source for society and the generation of diesel-ready hydrocarbons by cyanobacteria is evocative. Nonetheless, scaling production remains difficult and more costly than fossil fuels (23). New technologies are needed to develop competitive products as reflected by the transition from the US Department of Energy’s Aquatic Species Program (1978–1996) (9) to a new technology roadmap (24).
Biological hydrocarbon production is not unusual, but production by organisms as abundant as Prochlorococcus and Synechococcus is noteworthy. Whether or not their production scales with their numbers to the magnitude suggested in PNAS, the work of Lea-Smith et al. (12) provides useful insight into the nexus of biogeochemical cycles, petroleum pollution, and biofuel production for modern society.
Footnotes
The authors declare no conflict of interest.
See companion article on page 13591.
References
- 1.Hunt M. Petroleum Geochemistry and Geology. Freeman; New York: 1979. [Google Scholar]
- 2.Lerdau M, Guenther A, Monson R. Plant production and emission of volatile organic compounds. Bioscience. 1997;47(6):373–383. [Google Scholar]
- 3.Eglinton G, Gonzalez A, Hamilton R, Raphael R. Hydrocarbon constituents of the wax coating of plant leaves: A taxonomic survey. Phytochemistry. 1962;1(2):89–100. doi: 10.1038/193739a0. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Calvin M. Hydrocarbon distribution of algae and bacteria, and microbiological activity in sediments. Proc Natl Acad Sci USA. 1969;64(2):436–443. doi: 10.1073/pnas.64.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomquist G, Bagnères A, editors. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 6.Tornabene TG, Kates M, Gelpi E, Oro J. Occurrence of squalene, di- and tetrahydrosqualenes, and vitamin MK8 in an extremely halophilic bacterium, Halobacterium cutirubrun. J Lipid Res. 1969;10(3):294–303. [PubMed] [Google Scholar]
- 7.Chen J, Henderson G, Grimm CC, Lloyd SW, Laine RA. Termites fumigate their nests with naphthalene. Nature. 1998;392(6676):558–559. [Google Scholar]
- 8.Grice K, et al. New insights into the origin of perylene in geological samples. Geochim Cosmochim Acta. 2009;73(21):6531–6543. [Google Scholar]
- 9.Sheehan J, Dunahay T, Benemann J, Roessler P. 1998. A look back at the U.S. Department of Energy’s aquatic species program: Biodiesel from algae (National Renewable Energy Laboratory, Golden, CO)
- 10.Blumer M, Guillard RRL, Chase T. Hydrocarbons of marine phytoplankton. Mar Biol. 1971;8(3):183–189. [Google Scholar]
- 11.Schirmer A, Rude M, Li X, Popova E, DelCarayre S. Microbial biosynthesis of alkanes. Science. 2010;329(5991):559–562. doi: 10.1126/science.1187936. [DOI] [PubMed] [Google Scholar]
- 12.Lea-Smith DJ, et al. Contribution of cyanobacterial alkane production to the ocean hydrocarbon cycle. Proc Natl Acad Sci USA. 2015;112:13591–13596. doi: 10.1073/pnas.1507274112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flombaum P, et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci USA. 2013;110(24):9824–9829. doi: 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Energy Information Administration 2015 International Energy Statistics. Available at www.eia.gov/cfapps/ipdbproject/iedindex3.cfm?tid=5&pid=53&aid=1. Accessed September 29, 2015.
- 15.National Research Council . Oil in the Sea III: Inputs, Fates, and Effects. National Academies; Washington, DC: 2003. [PubMed] [Google Scholar]
- 16.Atlas E, Giam C. Sea-air exchange of high molecular weight synthetic organic compounds: Results from the SEAREX Program. In: Duce R, editor. Chemical Oceanography V10. Academic; New York: 1989. pp. 339–378. [Google Scholar]
- 17.Gagosian RB, Peltzer ET. The importance of atmospheric input of terrestrial organic material to deep sea sediments. Org Geochem. 1986;10(4–6):661–669. [Google Scholar]
- 18.Gros J, et al. First day of an oil spill on the open sea: Early mass transfers of hydrocarbons to air and water. Environ Sci Technol. 2014;48(16):9400–9411. doi: 10.1021/es502437e. [DOI] [PubMed] [Google Scholar]
- 19.Aeppli C, et al. Recalcitrance and degradation of petroleum biomarkers upon abiotic and biotic natural weathering of Deepwater Horizon oil. Environ Sci Technol. 2014;48(12):6726–6734. doi: 10.1021/es500825q. [DOI] [PubMed] [Google Scholar]
- 20.Valentine RC, Valentine DL. Omega-3 Fatty Acids and the DHA Principle. CRC; Boca Raton, FL: 2009. [Google Scholar]
- 21.Biller SJ, et al. Bacterial vesicles in marine ecosystems. Science. 2014;343(6167):183–186. doi: 10.1126/science.1243457. [DOI] [PubMed] [Google Scholar]
- 22.Van Mooy BAS, et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature. 2009;458(7234):69–72. doi: 10.1038/nature07659. [DOI] [PubMed] [Google Scholar]
- 23.Van Beilen Why microalgal biofuels won’t save the internal combustion machine. Biofuels Bioprod Biorefin. 2012;6(3):41–52. [Google Scholar]
- 24. Fishman D, Majumdar R, Morello J, Pate R, Yang J, eds (2010) National algal biofuels technology roadmap (US Dept. of Energy, Washington, DC)