Fig. 5.
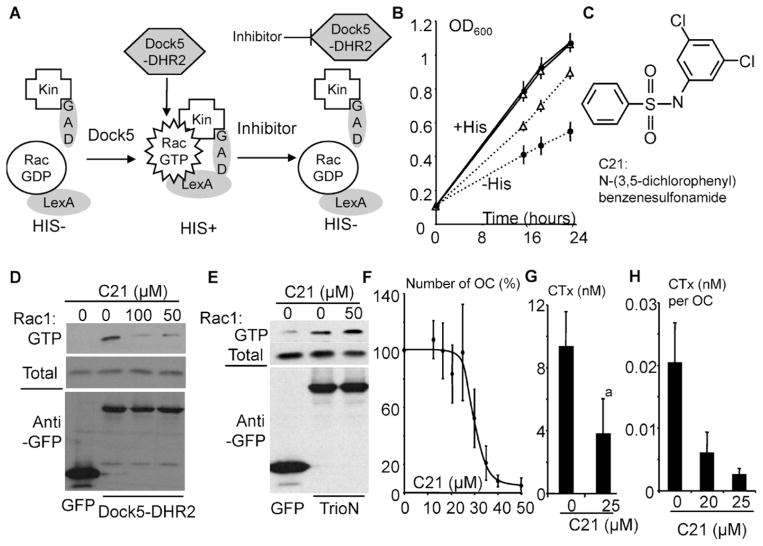
Inhibition of bone resorption by an inhibitor of Rac1 activation by Dock5. (A) Principle of the yeast exchange assay used to identify inhibitors of Dock5 exchange activity. Wild-type Rac1 is fused to LexA DNA-binding domain (LexA) and its effector kinectin (Kin) to the GAL4 activation domain (GAD). Expression of Dock5-DHR2 activates Rac1, which binds to kinectin, leading to expression of His3 and then yeast auxotrophy for histidine. Inhibitors of Dock5-DHR2 revert Rac1 activation. (B) Growth curves of yeasts expressing Rac1 and kinectin with (open triangles) and without (dots) Dock5-DHR2 in medium complemented (plain lines) or not (dotted lines) with histidine. (C) Structure of N-(3,5-dichlorophenyl)benzenesulfonamide (C21). (D) Western blots showing total and GTP-bound Rac1 (upper panels) and GFP and GFP-fused Dock5-DHR2 in extracts of 293T cells treated for 1 hour with the indicated concentrations of C21 in the presence of 1% DMSO. (E) Western blots showing total and GTP-bound Rac1 (upper panels) and GFP and GFP-fused TrioN in extracts of 293T cells treated as in panel D. (F) Number of OCs (OC) after a 24-hour incubation with the indicated concentrations of C21, expressed as a percent of DMSO control (0 μM C21). Graph shows average and SD of two to three independent experiments performed in triplicate. (G) CTX concentration in the medium of OCs on bone in the absence (0) or presence of 25 μM C21. Graph shows average and SD of three experiments performed in duplicate with independent OC preparations. ap <.001, Mann-Whitney test. (H) CTX production per OC in the presence of the indicated concentration of C21. Graph shows average and SD CTX concentration per OC in one experiment performed in triplicate.