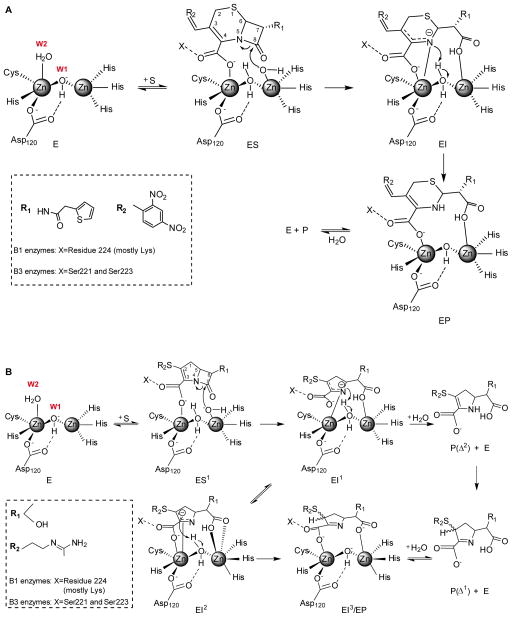
Figure 2.
A) Reaction mechanism for nitrocefin hydrolysis by di-Zn(II) B1 and B3 enzymes. EI is the experimentally characterized anionic intermediate interacting with Zn2 via the N atom and the carboxylate [66,91]. The same scheme would be valid for other chromogenic cephalosporins such as cromaceph and CENTA [92]. B) Reaction mechanism for imipenem hydrolysis by di-Zn(II) B1 and B3 enzymes. EI1 and EI2 are the experimentally characterized anionic intermediates [23], stabilized by interaction with Zn2. The representation of EI2 is based on the crystallographic structures of NDM-1 in complex with hydrolyzed meropenem (PDB 4EYL, see Figure 4) [44]. W1 stands for Wat1 and W2 stands for Wat2.