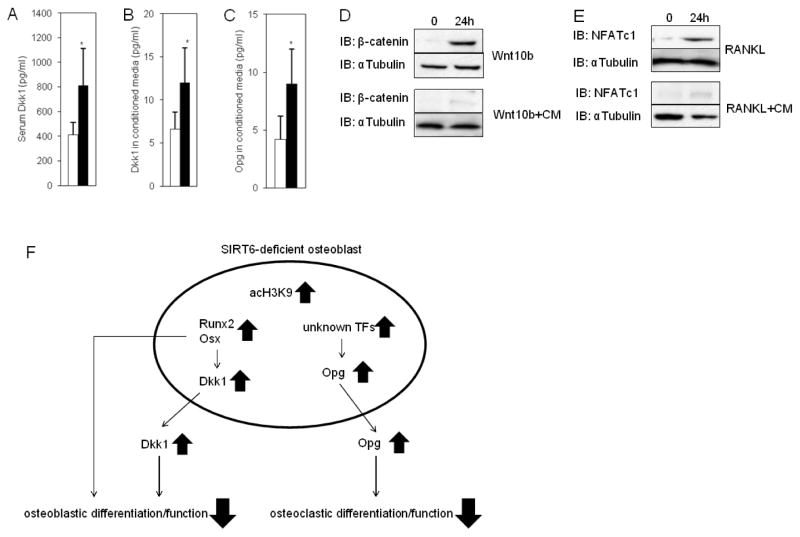
Fig. 6. Elevated Dkk1 and Opg levels in SIRT6−/− Osteoblasts.
(A) Elevated serum Dkk1 levels in SIRT6−/− mice. The data were expressed as the means ± S.D. of five mice of each genotype. *, p < 0.01. Open bar: WT, closed bar: SIRT6 deficiency. (B and C) Elevated Dkk1 or Opg levels in culture media of SIRT6−/− osteoblasts. Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (D) Western blots showing the nuclear accumulation of β-catenin in osteoblasts in response to Wnt10b with or without conditioned media (CM) harvested from SIRT6−/− osteoblast culture media. αTubulin was used as a loading control. (E) Western blots showing NFATc1 expression in splenocytes in response to RANKL with or without conditioned media (CM) harvested from SIRT6−/− osteoblast culture media. αTubulin was used as a loading control. (F) A model of a novel mechanism for controlling Dkk1 and Opg levels by SIRT6 in osteoblasts. Hyperacetylation of histone H3K9 on the promoters of Runx2 and Osx in SIRT6-deficient osteoblasts produces increased Runx2 and Osx levels so that Dkk1 levels are up-regulated that result in impaired osteoblastic differentiation and function. Opg levels are also increased by unknown mechanisms in SIRT6-deficient osteoblasts so that osteoclastic differentiation and function are diminished. This novel mechanism may trigger age-related bone loss. acH3K9: acetylated H3K9. TFs: transcription factors.