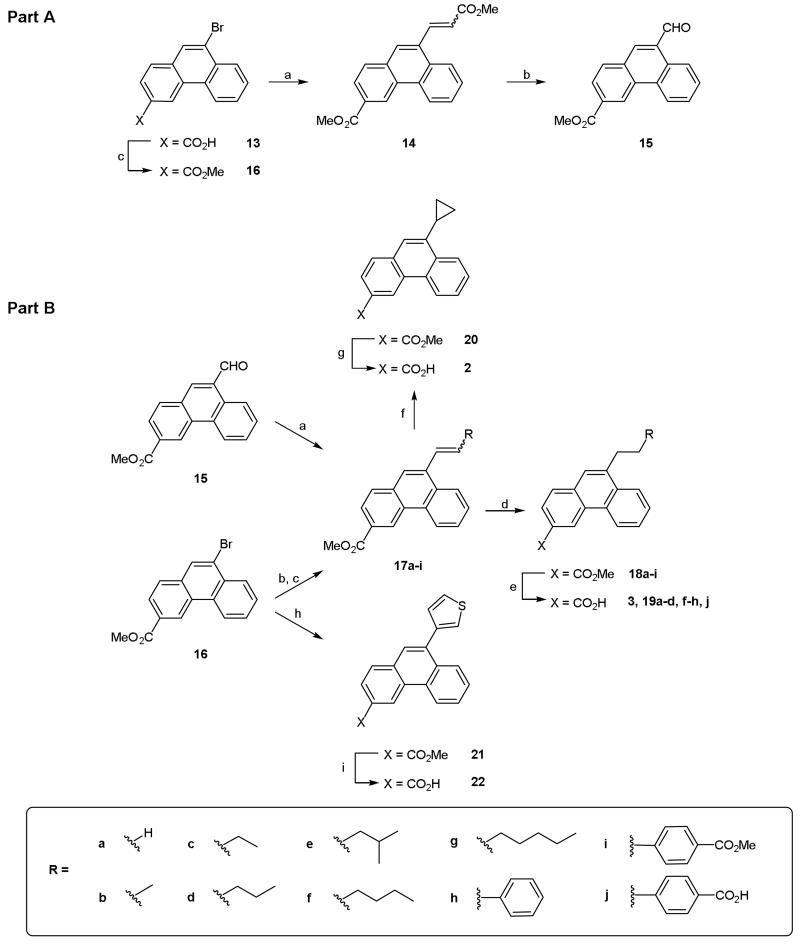
Scheme 3.
Reagents and conditions: Part A (a) (i) Methyl acrylate, P(o-tolyl)3, TEA, Pd(OAc)2, DMF, 100 °C, 18 h, (ii) MeI, K2CO3, DMF, rt, 18 h; (b) (i) OsO4, TMAO, t-BuOH/H2O, rt, 2 days, (ii) NaIO4; (c) MeOH, H2SO4, reflux, 48 h. Part B (a) RCH2PPh3X, KHMDS, THF, 4 h, rt; (b) alkene, P(o-tolyl)3, TEA, Pd(OAc)2, DMF, 100 °C, 18 h; (c) (n-Bu)3SnCH=CH2, Pd(PPh3)4, toluene, reflux, 4 h; (d) H2, 10% Pd/C, EtOAc, rt, 18 h; (e) (i) NaOH or KOH (aq), THF, reflux or dioxane, 75 °C, (ii) 1 M HCl (aq); (f) 17a, CH2I2, ZnEt2, DCM, 0 °C, 18 h; (g) (i) LiOH (aq), dioxane, rt, 18 h, (ii) 1 M HCl (aq); (h) 3-thienylboronic acid, K2CO3, Pd(dppf)Cl2.DCM, DME, 80 °C, 24 h; (i) (i) NaOH (aq), dioxane, 75 °C, (ii) 1 M HCl (aq), (iii) crystallisation (AcOH).