Figure 1.
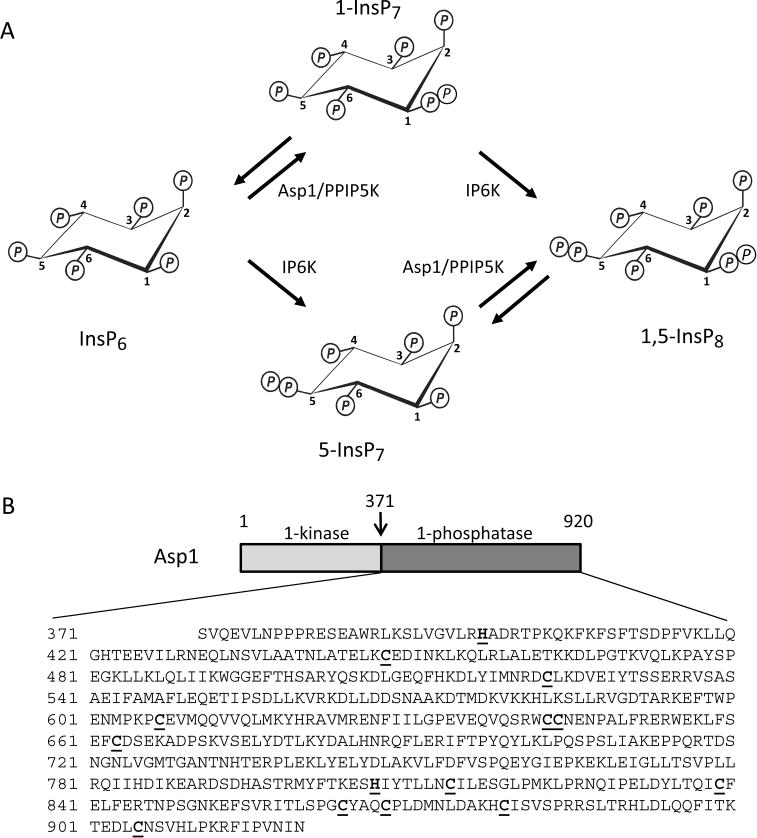
PP-InsP turnover by Asp1/PPIP5K. Panel A depicts the enzymatic reactions catalyzed by Asp1 (PIPP5K in mammals); see the Results and Discussion section for details. Panel B is a schematic of the domain structure of Asp1. A previously published domain alignment places Val375 at the N-terminus of the Asp1 phosphatase domain,9 but Ser371 is favored by our multiple sequence analysis (Supplementary Figure S1). Thus, we used Asp1371–920 as our phosphatase domain construct; its entire sequence is shown below the schematic. All Cys residues are underlined and in bold type. Similarly highlighted are two catalytically important His residues, His39711 and His807 (ref 9 and this work).
