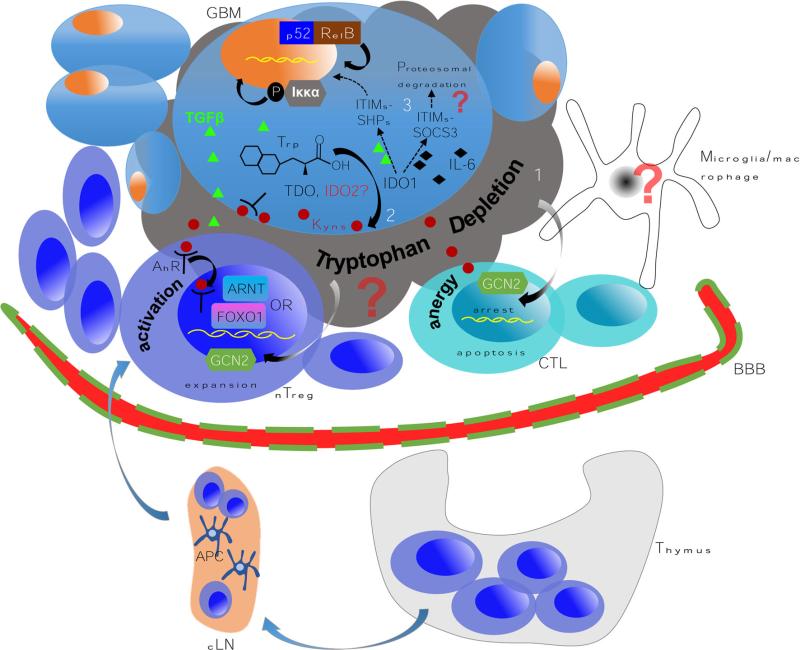
Fig. 4.
A paradigm for testing the multiple independent mechanisms that reinforce IDO1-mediated immunosuppression in malignant glioma. 1 The high expression of IDO1 leads to a commensurately high rate of tryptophan conversion and depletion. This induces cell cycle arrest and/or anergy in the effector (anti-glioma) T cell compartment via the eIF2α kinase GCN2-dependent pathway [39]. Simultaneously, the same mechanism activates Treg to become fully functional/mature in association with CTLA4:CD80/CD86 co-inhibition [23]. Although both microglia and macrophages can express IDO1, their contribution to this mechanism has yet to be established. 2 A complementary pathway mediated by IDO1 produces kynurenine that interacts with the aryl hydrocarbon receptor (AhR) leading to the expansion of glioma-resident Treg. Recent work has highlighted multiple interactions between the Ahr:Kyn. complex that may be required for Treg responsiveness. AhR interacts with the aryl hydrocarbon receptor nuclear translocator (ARNT) to mediate specific transcriptional programming [58] that may provide synergistic or independent impact to another recently identified heterodimeric partner, FOXO1 [59]. 3 The final known pathway that IDO1 utilizes to reinforce immunosuppression is via the two intrinsic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) [47]. Phosphorylation of ITIMs in IDO1 triggers a non-canonical NF-κB pathway, leading to phosphorylation of IKKα and nuclear translocation of the NF-κB subunits, p52 and RelB; reinforcing immunotolerance and TGF-β production. While it is still unclear whether all three of these mechanisms are actively involved in glioma, current studies in our laboratory aim to determine the various contributions