Figure 1. Roles of acetylation and SIRT3 in regulating mitochondrial functions.
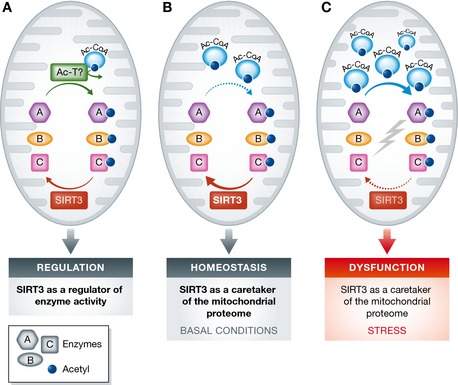
(A) A popular model posits that acetylation on mitochondrial proteins, regulated by SIRT3 and perhaps by putative mitochondrial acetyltransferases (Ac‐T), governs activities of various enzymes (designated A, B, and C) and flux through metabolic pathways, allowing mitochondria to adapt to varied cellular needs. This scheme would not predict a universally low stoichiometry of lysine acetylation in the mitochondria. (B) The results of Weinert and colleagues, and those of others, suggest that mitochondrial protein acetylation may occur largely as a passive consequence of non‐enzymatic reaction of proteins with acetyl‐CoA, and be efficiently removed by SIRT3 under most conditions in wild‐type animals. In this model, SIRT3 functions as a caretaker of the mitochondrial proteome, rather than as a regulator of enzyme activities in a strict sense. (C) Under stress conditions, mitochondrial acetyl‐CoA levels rise, and/or SIRT3 activity declines, allowing accumulation of acetylated proteins and consequently mitochondrial dysfunction. Such dysfunction might be amenable to correction by pharmacologic or genetic SIRT3 hyperactivity.
