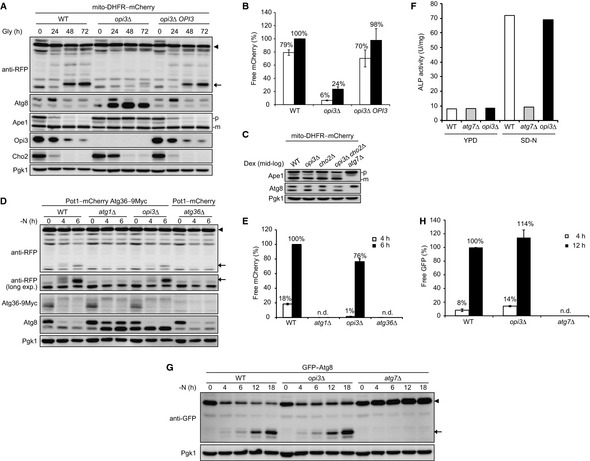
Reintroduction of an OPI3 gene into the opi3‐null mutant rescues mitophagy. Wild‐type, opi3Δ, and opi3Δ cells with a chromosomally reintroduced OPI3 gene expressing mito‐DHFR–mCherry (depicted by arrowhead) were grown in glycerol medium (Gly). Cells were harvested at the indicated time points and subjected to Western blotting. Generation of free mCherry (depicted by arrow) indicated transport of mitochondria to the vacuole. The cytosolic protein Pgk1 was monitored as a loading control.
The amounts of free mCherry detected in (A) were quantified at the indicated time points. The signal intensity value of free mCherry in wild‐type cells at the 72‐h time point was set to 100%. Data represent the averages of three experiments, with error bars indicating standard deviations.
Deletion of the OPI3 gene does not affect the Cvt pathway under fermentable conditions. Wild‐type, opi3Δ, cho2Δ, opi3Δ cho2Δ, and atg7Δ cells expressing mito‐DHFR–mCherry were grown in dextrose medium (Dex) and harvested at mid‐log phase. The vacuolar aminopeptidase Ape1 is synthesized as a precursor (p) in the cytosol, transported to the vacuole via the Cvt pathway, and processed into a mature form (m) in the vacuolar lumen. Pgk1 was monitored as a loading control.
Pexophagy is only slightly reduced in starving cells lacking Opi3. Wild‐type, atg1Δ, and opi3Δ cells expressing Pot1–mCherry (a peroxisome marker depicted by arrowhead) and Atg36–9Myc (a protein essential for pexophagy), and atg36Δ cells expressing Pot1–mCherry were grown in oleic acid medium, transferred to dextrose medium lacking nitrogen (−N), and harvested at the indicated time points. Generation of free mCherry (depicted by arrow) indicated transport of peroxisomes to the vacuole. Pgk1 was monitored as a loading control.
The amounts of free mCherry detected in (D) were quantified at the indicated time points. The signal intensity value of free mCherry in wild‐type cells at the 6‐h time point was set to 100%. Data represent the averages of three experiments, with error bars indicating standard deviations.
Opi3 is dispensable for autophagy. Wild‐type, atg7Δ, and opi3Δ cells expressing Pho8Δ60 (a cytosolic alkaline phosphatase for monitoring autophagy activity) pre‐grown in nutrient‐rich dextrose medium (YPD) were incubated in synthetic dextrose medium lacking nitrogen (SD‐N) for 6 h and subjected to alkaline phosphatase (ALP) assays. Pho8Δ60 is transported to the vacuole via autophagy and processed into an active form in the vacuolar lumen.
Starvation‐induced autophagy flux is normal in the absence of Opi3. Wild‐type, opi3Δ, and atg7Δ cells expressing GFP–Atg8 (depicted by arrowhead) were grown to mid‐log phase in rich dextrose medium, incubated for the indicated time points in starvation medium (−N), and subjected to Western blotting. Generation of free GFP (depicted by arrow) indicates transport of autophagosomes to the vacuole. Atg7 is an E1 enzyme essential for autophagy. Pgk1 was monitored as a loading control.
The amounts of free GFP detected in (G) were quantified at the 4‐ and 12‐h time points. The signal intensity value of free GFP in wild‐type cells at the 12‐h time point was set to 100%. Data represent the averages of three experiments, with error bars indicating standard deviations.