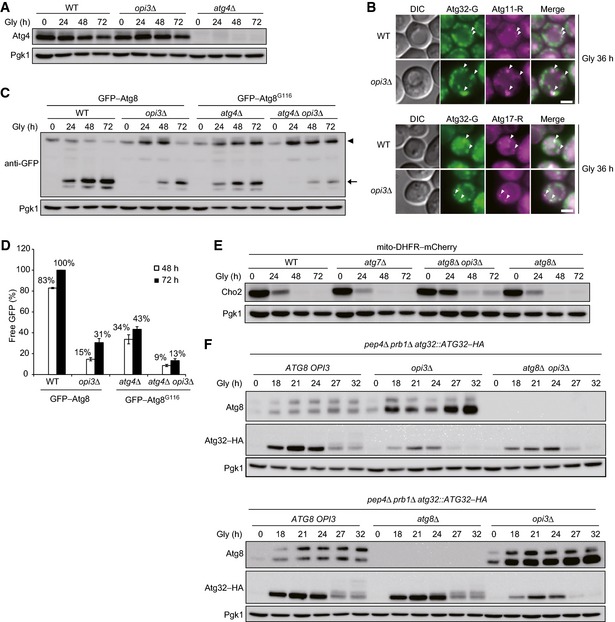
Atg4 expression is not altered in the absence of Opi3. Wild‐type, opi3Δ, and atg4Δ cells expressing mito‐DHFR–mCherry were grown in glycerol medium (Gly), harvested at the indicated time points, and subjected to Western blotting. Pgk1 was monitored as a loading control.
Atg32 properly localizes to the pre‐autophagosomal structure (PAS) in opi3‐null cells. Wild‐type and opi3Δ cells expressing Atg32–GFP (Atg32–G) and Atg11–2xmCherry (Atg11–R) or Atg17–2xmCherry (Atg17–R) were grown in glycerol medium (Gly) for 36 h and observed under a fluorescence microscope. Atg11–2xmCherry and Atg17–2xmCherry were used as PAS markers. Arrowheads indicate Atg32–GFP colocalized with Atg11–2xmCherry or Atg17–2xmCherry. Scale bar, 3 μm.
Deletion of the OPI3 gene leads to a strong reduction in autophagy flux. Wild‐type or opi3Δ cells expressing GFP–Atg8 (depicted by arrowhead), and atg4Δ or atg4Δ opi3Δ cells expressing GFP–Atg8G116 (depicted by arrowhead) were generated from a strain for mitophagy assays, grown in glycerol medium (Gly), and harvested at the indicated time points. Generation of free GFP (depicted by arrow) indicated transport of autophagosome‐related vesicles to the vacuole. Pgk1 was monitored as a loading control.
The amounts of free GFP detected in (C) were quantified at the indicated time points. The signal intensity value of free mCherry in wild‐type cells at the 72‐h time point was set to 100%. Data represent the averages of three experiments, with error bars indicating standard deviations.
Cho2 repression is retarded in opi3‐null cells even without Atg8 lipidation. Wild‐type, atg7Δ, atg8Δ opi3Δ, and atg8Δ cells expressing mito‐DHFR–mCherry were grown in glycerol medium (Gly) and harvested at the indicated time points. Pgk1 was monitored as a loading control.
Loss of Opi3 suppresses Atg32 induction independently of Atg8 lipidation. Wild‐type, opi3Δ, and atg8Δ opi3Δ cells expressing chromosomally encoded Atg32–HA were generated from a vacuolar protease‐deficient strain (pep4Δ prb1Δ), grown in glycerol medium (Gly), harvested at the indicated time points, and subjected to Western blotting. Pgk1 was monitored as a loading control.