Figure EV4. Yeast and human Atg8 family proteins are conjugated to PMME in vitro .
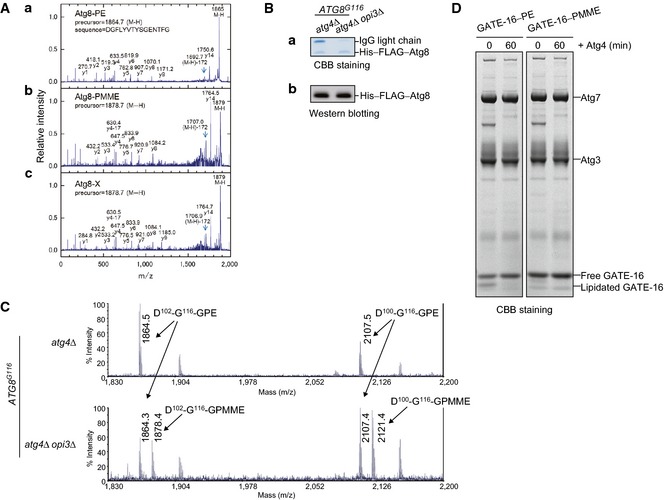
- Atg8 is conjugated to PMME in cells lacking Opi3. Membrane‐enriched fractions were isolated from opi3Δ cells expressing His–FLAG–Atg8, solubilized, and subjected to tandem affinity chromatography as in Fig 5C and D. Negative‐ion MALDI‐MS/MS spectra of the C‐terminal peptide of His–FLAG–Atg8 from opi3Δ cells (c) was compared with those of in vitro‐generated Atg8–PE (a) and Atg8–PMME (b).
- Proteins were purified from atg4Δ or atg4Δ opi3Δ cells expressing His–FLAG–Atg8G116 as in Fig 5C and analyzed by SDS–PAGE and CBB staining (a) or Western blotting (b).
- Atg8G116 is conjugated to PE and PMME in atg4 opi3‐null cells. Negative‐ion MALDI‐MS spectra of the C‐terminal peptides of His–FLAG–Atg8G116 purified as in (B). The C‐terminal fragments were generated by saponification and protease treatment.
- GATE‐16 is conjugated to PMME but cannot be efficiently delipidated in vitro. Lipidation reaction of the human Atg8 homolog GATE‐16 was performed for 2 h as in Fig 5A and B. The reactions were further incubated with Atg4 for the indicated time periods. Samples were then analyzed by Bis‐Tris NuPAGE and Coomassie Brilliant Blue (CBB) staining.
