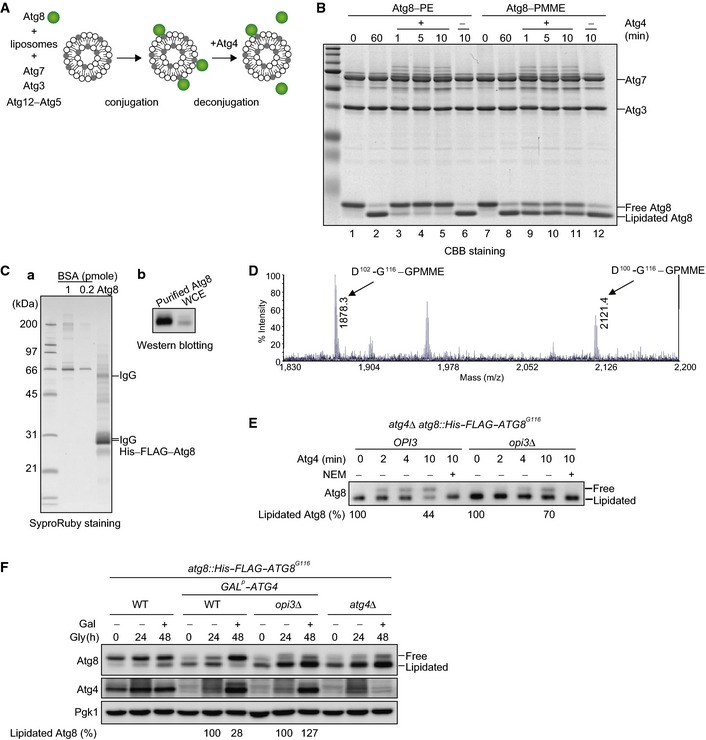
A schematic diagram of Atg8 conjugation and deconjugation reactions in vitro. Atg8G116, an activated variant lacking the C‐terminal arginine, was incubated with liposomes containing PE or PMME in the presence of Atg7 (E1), Atg3 (E2), Atg12–Atg5 (E3‐like conjugate), and ATP. The Atg4 cysteine protease was then added to the reactions for Atg8 delipidation.
Atg8 lipidation reaction was performed for 60 min as in (A). The reactions were further incubated with Atg4 or mock‐treated for the indicated time periods. Samples were then analyzed by urea–SDS–PAGE and Coomassie Brilliant Blue (CBB) staining.
Membrane‐enriched fractions were isolated from opi3Δ cells expressing His–FLAG–Atg8, solubilized, and subjected to tandem affinity chromatography. Proteins were then eluted with SDS‐sample buffer and analyzed by SDS–PAGE and SyproRuby staining (a), or urea–SDS–PAGE and Western blotting (b). Bovine serum albumin (BSA) was used as a standard.
Negative‐ion MALDI‐MS spectrum of the C‐terminal peptide of His–FLAG–Atg8 expressed in cells lacking Opi3. His–FLAG–Atg8 was purified as in (C), and the C‐terminal fragments were generated by saponification and protease treatment. The GPMME‐modified peptide, observed at m/z 1878.3, was further subjected to MS/MS (see Fig
EV4A).
In vitro Atg4 processing assays. Membrane‐enriched fractions were isolated from atg4Δ or atg4Δ opi3Δ cells expressing His–FLAG–Atg8G116 and treated with Atg4 for the indicated time periods in the presence or absence of N‐ethylmaleimide (NEM), a cysteine protease inhibitor. Samples were then purified and analyzed as in (C).
Wild‐type or atg4Δ cells expressing His‐FLAG‐ATG8G116, and wild‐type or opi3Δ cells expressing His‐FLAG‐ATG8G116 with the ATG4 gene under the GAL promoter were grown in glycerol medium (Gly) for 24 h, supplemented with galactose (Gal), and subjected to Western blotting. The amounts of lipidated Atg8 detected in wild‐type or opi3Δ cells containing the GAL‐driven ATG4 gene were quantified at the 24‐ and 48‐h time points. The signal intensity values of lipidated Atg8 in wild‐type and opi3Δ cells at the 24 h time point were set to 100%. Pgk1 was monitored as a loading control.