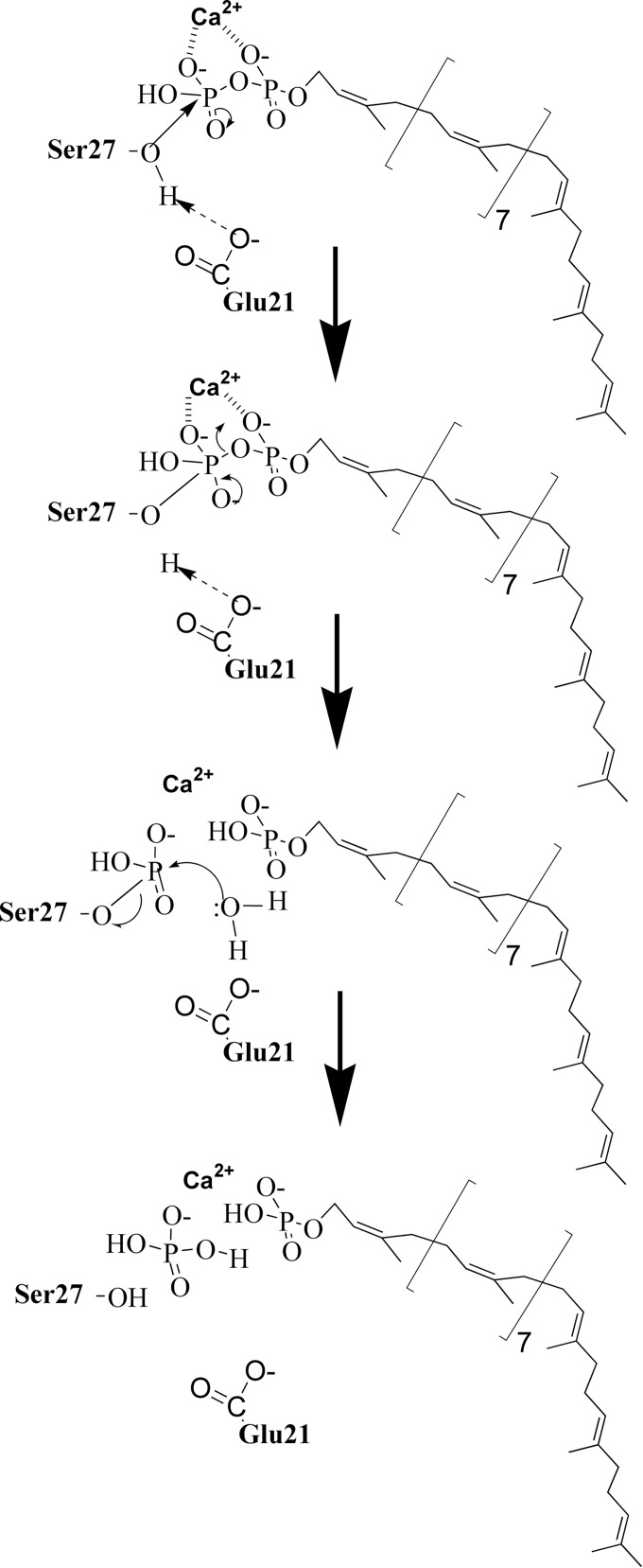
Fig 6. Proposed catalytic mechanism for the BacA C55-PP phosphatase.
The present mechanism is based on the results of the structure-activity relationship analysis developed in this work. Two residues Glu21 and Ser27 that are essential for the catalytic process were identified, whose mutation into alanine yielded BacA enzymes with dramatically altered activities (1.4% and 0.013% of residual activity, respectively). The Ser27 residue has a hydroxyl group that can act as a nucleophile when deprotonated by the carboxyl group of Glu21 residue, the latter group displaying a negative charge at the pH that is optimal for BacA activity. The deprotonated Ser27 then initiates a nucleophilic attack on the β-phosphorus center of C55-PP substrate to form a phospho-serine enzyme intermediate, a reaction releasing the C55-P product. A subsequent attack by a water molecule releases the second inorganic phosphate product.