Abstract
Aquatic environments can be restricted with the amount of available food resources especially with changes to both abiotic and biotic conditions. Mosquito larvae, in particular, are sensitive to changes in food resources. Resource limitation through inter-, and intra-specific competition among mosquitoes are known to affect both their development and survival. However, much less is understood about the effects of non-culicid controphic competitors (species that share the same trophic level). To address this knowledge gap, we investigated and compared mosquito larval development, survival and adult size in two experiments, one with different densities of non-culicid controphic conditions and the other with altered resource conditions. We used Aedes camptorhynchus, a salt marsh breeding mosquito and a prominent vector for Ross River virus in Australia. Aedes camptorhynchus usually has few competitors due to its halo-tolerance and distribution in salt marshes. However, sympatric ostracod micro-crustaceans often co-occur within these salt marshes and can be found in dense populations, with field evidence suggesting exploitative competition for resources. Our experiments demonstrate resource limiting conditions caused significant increases in mosquito developmental times, decreased adult survival and decreased adult size. Overall, non-culicid exploitation experiments showed little effect on larval development and survival, but similar effects on adult size. We suggest that the alterations of adult traits owing to non-culicid controphic competition has potential to extend to vector-borne disease transmission.
Introduction
The effectiveness of exploitative competition for available food resources is driven by the presence of species within the same trophic level (controphic species) and species that share the same resources and functional feeding group [1, 2]. For mosquitoes, the presence of other species that are filter- and suspension-feeders can limit the amount of available food [3]. Consequently, the outcomes of controphic resource (exploitative) competition on mosquito development and survival should be equivalent to increased intra-specific resource limitation; with greater effects on mosquito development, survival and adult size with increasing competition. However, this hypothesis is yet to be investigated.
Over the last two decades, significant effort has been directed to understanding the diverse effects that biotic interactions have on the ecology of mosquito vectors [1, 4–7]. The larval life-stages of mosquitoes are the most sensitive to biotic interactions with impacts on developmental times, survival or changes to adult size. These impacts can, in turn, effect fecundity and, for vectors of disease, vector competence capacity [8–10]. To date, investigations have largely focused on larval predation, inter- and intra- specific mosquito competition (particularly with Aedes aegypti (Linnaeus) and Aedes albopictus (Say)) and mosquito oviposition behaviour as a means of predation and competition avoidance [11, 12]. The importance of competitive interactions with other non-culicid invertebrates, particularly non-culicid controphic species is poorly understood, yet may play an important role in mosquito abundance within natural conditions, with consequent implications for vectors [13, 14].
Across southern Australia Ae. camptorhynchus (Thomson) [15, 16] is a major vector of Ross River virus (RRV: Togaviridae: Alphavirus). Epidemiologically, RRV is Australia’s most important vector-borne disease with clinical notifications ranging between 1451 – 7754 per annum, resulting in an annual economic impact of approximately $15 million dollars [17]. Salt marshes are particularly important habitats for this halo-tolerant vector due to the hyper-saline aquatic conditions. Such physiologically extreme environments result in lower aquatic species richness, and hence fewer predators and competitors of Ae. camptorhynchus, which is indicative of regions where this mosquito occurs across Australia [15, 18–20]. In these habitats Ae. camptorhynchus lays desiccation resistant eggs that undergo mass hatching with large pulses of rainfall or tidal inundations [21, 22]. Likewise, these pulses of water also result in high densities of micro-crustaceans that are capable of surviving dry periods [23, 24]. The Diacypris spp. (Crustacea: Ostracoda) that dominate the remaining aquatic fauna in these environments [25] are detritovores/herbivores, occupying the same functional feeding group as Ae. camptorhynchus. In addition, a negative density relationship from field evidence suggests these species may interact via exploitative competition [26]. If exploitative competition is occurring between these species we expect it will impact mosquito vector development, survival, abundance and size [26].
In this study we test the hypothesis that non-culicid controphic exploitative competition with ostracods reflects intra-specific resource limitation. The reciprocal of our hypothesis is that exploitative competition is not occurring between these taxa and ostracods will therefore have no impact on Ae. camptorhynchus development and survival. We examined our hypothesis using two experiments: an intra-specific resource limitation experiment with low densities of Ae. camptorhynchus; and a non-culicid controphic exploitative competition experiment with ostracods in increasing densities. Between the two experiments we contrast the changes in larval developmental times and survival, and effects on adult size. For both experiments, we predict decreased larval survival, increased development time, and reduced adult size as resources become limiting and controphic competition increases. We observed development and survival changes with intra-specific resource limitation and changes in adult size for both intra-specific resource limitation and controphic competition treatments. Our findings suggest no evidence of exploitative competition among Ae. camptorhynchus and ostracods during larval development, but detectable similarities to resource limitation with adult size.
Materials and Methods
Invertebrate collections
All mosquitoes and ostracods used in this study were sourced from the Primrose Sands salt marsh (147°39 East, 42°52 South), east of Hobart, Tasmania, whereby permission to access the marsh was granted by the land owner. Water bodies found in the Primrose Sands salt marsh are very ephemeral, lasting on average only 14 days after inundation over the peak of summer [27]. Such environments are very depauperate of aquatic species diversity as shown by Carver et.al. [26] where, after rainfall, 89% of faunal abundance comprised of Ae. camptorhynchus (56%) and ostracods (33%), increasing to 91% in drying conditions (Ae. camptorhynchus 46% and ostracods 45%) [28]. Examination of the aquatic community strongly indicates that the ostracod, Diacypris spp. (Crustacea:Ostracoda) [25], is the only plausible competitor for resources with Ae. camptorhynchus and was, therefore, used in the competition experiment. Mosquitoes and ostracods were collected either after substantial rainfall or tidal inundations that submerged most of the salt marsh which provided newly hatched Ae. camptorhynchus larvae (1st instars, < 24 hours old). While every attempt was made to collect early first mosquito instars, age was difficult to control in practice, so for consistency examination of developmental rates in this study are restricted to the second instar onwards. All invertebrates were collected using a 350 mL plastic larval dipper (Australian Entomological Supplies Pty. Ltd.).
Laboratory conditions
Following field collections, mosquito larvae were placed into 500 mL translucent cylindrical plastic containers with 200 mL of water at 35 ppt salinity (“Red Sea Salts”; at 35 ppt elements are 8.2–8.4 pH, 7.8–8.3 Alk (dKH), 420–440 Ca (mg/L), 1250–1310 Mg (mg/L) and 380–400 K (mg/L)), with containers placed randomly in temperature cabinets (Andrew Thorn Limited Qualtex 68 R4) at 23°C ± 0.05 S.E., 14:10 day/night. The temperature and salinity were chosen for laboratory conditions based on the average summer daily temperatures (°C) and salinity (ppt) of water bodies in the field near Hobart [27].
It is difficult to know the exact nutrient variables most relevant to mosquitoes within salt marshes and this is an important area for future studies. As a consequence, invertebrate food consisted of ground “Nutrafin Max Fish Flakes” (Pets Domain). Four grams were ground using a mortar and pestle and suspended in 1 L distilled water. At each feed the solution was agitated to allow for homogeneity and refrigerated at 6°C between feeds to standardise the potential growth of microbes. Although not entirely analogous to their field based diets, food levels and type were representative of other laboratory studies of culicid nutrition and development [29–31] and helped standardise nutritional quality and quantity which could otherwise vary if using field collected resources. New food was prepared fortnightly.
Experimental design
We conducted a paired experimental design to examine the effects of both intra-specific resource limitation and non-culicid exploitative competition on mosquito development and survival. While a fully crossed design would have been optimal, this was beyond the scope of the study owing to the logistics of available incubators and number of ostracods required. Instead this study provides a paired design where intra-specific resource limitation and non-culicid controphic exploitative competition experiments are contrasted. In the resource limitation experiment 50 larvae were exposed to one of four food resource levels (0.1 mL, 0.2 mL, 0.4 mL or 0.6 mL food/day), with six replicate cylinders per treatment. For the exploitative competition trials 50 larvae were exposed to one of four treatments of competitor (ostracod) density (0 (control), 150, 300 and 600 ostracods/cylinder). This reflects the observed range of ostracod densities per 350 mL larval dippers (Australian Entomological Supplies) in water bodies at Primrose Sands between 2011 and 2012 (1–2144, n = 442) [27]. Food resources remained at a constant level of 0.4 mL/day. Each treatment consisted of 10 replicate cylinders, with the non-culicid exploitation control treatment being comparable to the 0.4 mL/day treatment in the resource experiment. Two replicates from each treatment from both experiments were dispersed evenly among three (resource experiment) or five (non-culicid exploitation experiment) independent incubators (akin to blocks), with the position of replicates randomised within each incubator.
Daily counts of mosquito larvae included the number of larvae, instar of each larva, number of pupae and number and sex of adults. Any mosquito larvae or pupae that died were recorded and removed from the container. All emergent mosquitoes were collected, sexed and their wings removed and mounted using water onto glass slides and sealed with clear nail varnish. The average length of left and right wings were used as a proxy for adult size [32]. These were measured at 25× magnification from the wing tip (excluding the fringe) to the arculus [10, 33] using Las EZ software (Leica Microsystems, Switzerland). Developmental time for instars–pupae in each replicate container was as the day at which 50% of surviving larvae reached the next stage of development.
To account for size variation of ostracods among treatments, a subsample of five ostracods from each controphic resource limiting replicate was removed at the beginning of the experiment and again at the end of the experiment (when the last mosquito emerged or died in each replicate container). Measurements of the carapace, from posterior to anterior, were conducted using an ocular micrometer (0.016mm units per graticule unit) on a Nikon SM2800 dissecting microscope at × 6.3 magnification. All remaining ostracods in each replicate were scored for survival.
Analyses
In both experiments we evaluated how treatment affected larval developmental times, survival, and adult size. Larval development was determined as developmental time when 50% of surviving larvae had reached the next each instar stage, pupa or adult. Survival was measured as the number of emerging adults and size was based on wing length measurements. An alternative approach to evaluating survival is by Cox hazard models, but we could not reliably identify individual survival in our experiment design, precluding this type of analysis. We used a Bayesian mixed effects modelling approach, with incubator as the random effect, owing to its superior ability to estimate coefficients than non-bayesian approaches, in a mixed effects framework [34]. In each model Y ij (larval developmental time, survival and adult size) was measured for each replicat cylinder i = 1,…, n j for incubator j = 1,…, k. The distribution of Y among replicates was assumed to have a Gaussian distribution with parameter π ij:
where π ij is the modelled Y of replicate i in incubator j. We modelled the Y, π ij, based on the effects of treatment
where α and β are the model intercept and slope, respectively, for replicate i varying by incubator j, and x was the assigned experimental treatment (intra-specific resource or non- culicid controphic exploitative resource limiting level) for replicate i. An additional fixed effect of sex was included for the model of adult size. Prior distributions for all model parameters in the hierarchy (incubators) were given with the goal of providing conjugate priors that contain little to no influence on the posterior distributions of all the model parameters. We assumed normal prior distributions on slopes, α, and intercepts, β, with mean μ and variance σ 2:
For the variance parameters, σ 2, we determined and utilized non-informative uniform prior hyper-parameter distributions, specified as σ 2~Uniform (0, 100), which was used across all models. Models were fitted in R (v 3.0.3) [35] using the ‘MCMCglmm’ package [36], with MCMC chains run for 13,000 iterations after a burn-in period of 3,000 iterations, ensuring convergence of model parameters, assessed following Gelman and Hill [34]. We summarized posterior distributions of model coefficients, β, by the Bayesian median and 95% credible intervals and MCMC simulated P-values.
We assessed consistency of results between the two experiments at the 0.4 mL/day intra-specific resource limiting treatment and the control treatment (no ostracods and same food level) from the non-culicid resource exploitation experiment. This was undertaken for development, survival and adult size using a Bayesian mixed effects model as described above, but with experiments being the fixed effect.
Using a Bayesian mixed effects model as previously described, we also investigated ostracod size (measured through sub-samples) and mortality amongst competition treatments (end count of ostracods) to explore if these factors changed Ae. camptorhynchus developmental times, survival and size or ostracod development and mortality. This analysis was undertaken for quality control purposes, as changes in these could confound non-culicid exploitative competition treatment effects.
Results
Development time
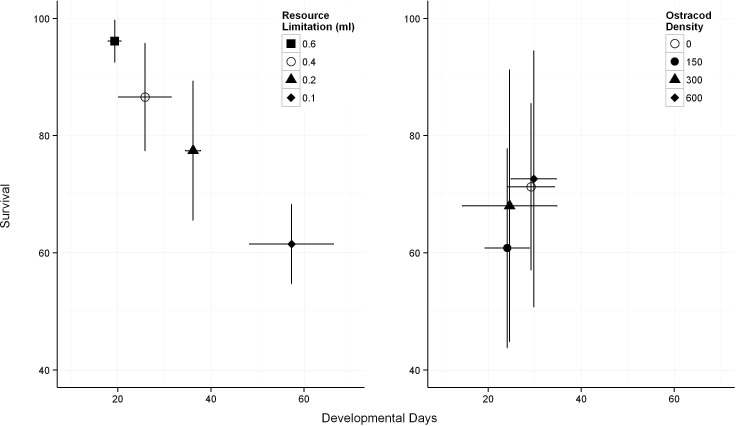
As the amount of intra-specific resources increased the time it took for Ae. camptorhynchus to develop decreased (Table 1). This relationship was observable within each larval stage with the exception of the pupal stage when mosquitoes do not feed. Overall, the mean developmental time of Ae. camptorhynchus to adult was 35 days with a range of 21–70 days (Fig 1; S1 Table). In contrast, the time taken for Ae. camptorhynchus larvae to develop was unrelated to the number of competitors. The only significant effect detected was for third instar which developed more slowly with increasing numbers of ostracods (Table 1). Mean time to develop to adult was 27 days ranging between of 33–39 days (Fig 1; S1 Table). When comparing the two experiments, there was no difference in developmental time between the 0.4mL/day intra-specific resource limitation treatment and the control treatment (no ostracods and same food level) for the non-culicid exploitation competition experiment (p = 0.176) (Fig 1).
Table 1. Bayesian mixed-effects regression results showing effects (median coefficient and 95% credible intervals) of both resource limitation and exploitation competition treatments on Ae. camptorhynchus larval development time (in days) between instar, pupae and total developmental time to adulthood, the effect of treatment on survival and the effect of both treatment and sex on adult wing length (mm) for both resource limitation and competition experiments. Significant p values are in bold.
| Experiment | Resources | Competition | ||||||
|---|---|---|---|---|---|---|---|---|
| coefficient | 2.5%CI | 97%CI | p | coefficient | 2.5%CI | 97%CI | p | |
| Development (days) | ||||||||
| Treatment effect on larvae | ||||||||
| Second instar | -0.710 | -1.169 | -0.189 | 0.006 | -4.74e-5 | -2.15e-3 | 1.55e-3 | 0.984 |
| Third instar | -3.379 | -4.594 | -1.789 | <0.001 | 0.002 | 0.260–3 | 0.003 | 0.008 |
| Fourth instar | -1.431 | -2.405 | -0.293 | 0.004 | 0.003 | -0.002 | 0.008 | 0.262 |
| Pupae | 0.036 | -0.137 | 0.207 | 0.654 | 0.002 | -0.003 | 0.008 | 0.398 |
| Overall | -6.893 | -8.658 | -5.205 | <0.001 | 0.003 | -0.007 | 0.013 | 0.55 |
| Survival | ||||||||
| Treatment | 11.257 | 8.088 | 14.201 | <0.001 | 0.006 | -0.024 | 0.036 | 0.696 |
| Wing length (mm) | ||||||||
| Treatment | 0.069 | 0.061 | 0.077 | <0.01 | <-0.001 | <-0.001 | <-0.001 | <0.01 |
| Sex | 0.038 | 0.007 | 0.068 | 0.02 | -0.093 | -0.037 | 0.017 | 0.526 |
Fig 1. Effects of resource limitation (left panel) and exploitation competition (right panel) on Ae. camptorhynchus mean development time and survival to adulthood (mean ± SD).
Treatments with open symbols are directly comparable.
Survival
Aedes camptorhynchus survival decreased as intra-specific resources became more limited (Table 1). The mean range of survival was between 61.4% – 96.1% (Fig 1; S1 Table). By comparison, increasing non-culicid exploitative competition did not affect Ae. camptorhynchus survival (Table 1), with a mean range of survival between 60.8% – 72.6% (Fig 1; S1 Table). Comparing the two experiments survival was slightly higher in the resource limitation than the control treatment (no ostracods and same food level) for non-culicid exploitative competition experiments (p = 0.028) (Fig 1).
Adult size
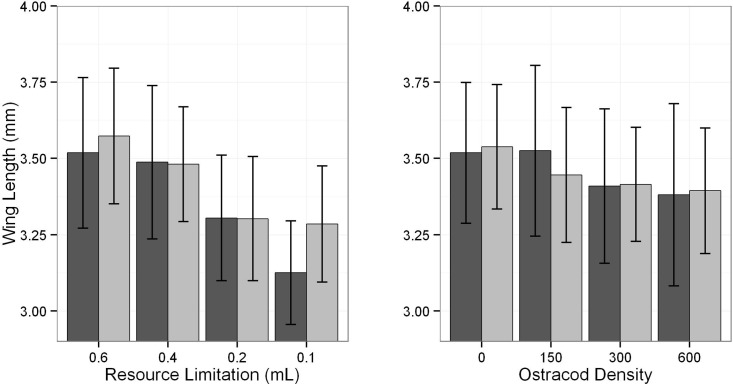
The size of Ae. camptorhynchus (based on wing length) declined as intra-specific resources became more limiting (Table 1). Overall, emerging adults were 9.86% larger in the most resource rich treatment, relative to all the other resource treatments (Fig 2). There was also a sex-specific effect on size with the wings of males being 0.15 mm larger than females, however this was only in the most limiting resource treatment. Likewise, increased non-culicid controphic competitors resulted in decreased size of Ae. camptorhynchus adults (Table 1). On average adults emerging from the control (no ostracods) were 4% larger than adults from the highest ostracod density treatment (Fig 2). There was no significant sex-specific size difference between treatments owing to ostracods (Table 1). Comparing the two experiments, adult size did not differ (p = 0.91, Fig 2).
Fig 2. Mean (± SD) wing length (mm) for Ae. camptorhynchus adult females (dark grey) and males (light grey) for both resource limitation (left panel) and competition (right panel) treatments.
Ostracod mortality and size
Ostracods within the non-culicid controphic exploitative treatments averaged 0.842 mm in length with no significant difference in size over the duration of the experiment (P = 0.624). Similarly, ostracod survival did not significantly differ between experimental treatments (P = 0.628), with an overall average 17% loss of ostracods.
Discussion
Like resource limitation, competitive interactions can affect mosquito life history, thereby potentially having an important role in the ecology of disease vectors. The results of our intra-specific resource limitation experiments are consistent with previous work that has investigated the effects of resource limitation on mosquito development and survival [31, 37–40] showing decreased developmental times, decreased survival and reduced adult sizes as resources become more scarce. However, when numbers of ostracod controphic competitors are increased, at least in our experimental conditions, exploitative competition had no impact on larval Ae. camptorhynchus developmental traits, but do effect adult size. Adult body size decreased as the density of ostracods increased, suggesting exploitative competition among Ae. camptorhynchus and ostracods may cause some effects similar to resource limitation. Our study is the first to directly test non-culicid controphic competition with ostracods, which form the same functional feeding group as Ae. camptorhynchus. We demonstrate that this interaction is an important component to mosquito ecology providing insight into the complexity of aquatic interactions with an important vector species.
As expected, reducing the resources available to Ae. camptorhynchus drove increased developmental times [31, 38–41] likely through intra-specific competition. Similar extension of developmental time has been demonstrated for both Ae. albopictus (Skuse) and Ae. aegypti in limiting diets and this was inferred to indicate impacts on accumulation, assimilation and storage of energy gained from food resources [42]. Prolonged larval developmental times could have negative impacts in the field, especially in conditions where water bodies are extremely ephemeral such as salt marshes. For example, extended developmental durations in these aquatic habitats increase exposure to habitat loss through water bodies drying up [43], thereby having the potential to result in decreased population sizes and reduced disease transmission.
The effects on non-culicid controphic competition on mosquitoes is a relatively new field of research, with few studies detailing increased developmental times in the presence of such exploitative competitors [1, 4, 44]. In these situations, where resources are shared [1, 3, 24], it is common to expect exploitation competition of one species over the other [45, 46]. It was expected that our non-culicid exploitative experiment would show that increasing ostracod density would cause longer Ae. camptorhynchus development. This expectation was supported by other studies, such as Stav et al. [44] where the authors presented increased time to metamorphosis of Culex pipens (Linnaeus) in the presence of Daphnia magna (Straus). In our trials the only significant impact on development we observed was during the third instar, with no effect of ostracod density on overall developmental times. While it is plausible that the densities within our trials were inadequate to result in exploitative competitive outcomes on Ae. camptorhynchus life history, the number of ostracods used in these trials reflect ostracod densities found in natural water bodies [26, 27]. Therefore, given that Ae. camptorhynchus maintained consistent rates of development across density treatments of ostracods, and ostracod mortality was not significant across treatments, we suggest exploitative competition is unlikely to be limiting larval development in our system.
Similarly, our intra-specific resource limiting trials showed Ae. camptorhynchus survival declined as resources became limited, but Ae. camptorhynchus survival did not change with increasing densities of ostracod competitors. Declines in mosquito survival has been documented in other non-culicid controphic competitive studies [1, 13], for example, Mokany and Shine [13] demonstrated a decline in Aedes australis survival as a result of interference competition between tadpoles (Limnodynastes peronii), however this was a result of chemical interference not exploitative competition. In contrast, Daugherty and Juliano [47] demonstrated improved survival of Ae. triseriatus in the presence of higher densities of scirtid beetles, because of the quantities of faeces excreted by the scirtids supported microorganisms that nourished the developing mosquito larvae. This, again, is not exploitative competition. Overall, our results suggest that, like developmental rates, the density of ostracods has little impact on mosquito survival through exploitative competition in this system.
Given the lack of detectable effects of ostracod density on Ae. camptorhynchus larval development and survival, it was somewhat surprising to see an effect on adult size. Limiting resources is known to cause emerging mosquitoes to be smaller [31]. For example, Ae. agypti adults emerging from nutrient deprived crowded conditions were significantly smaller. Here we suggest ostracod competitors may have a similar effect. Therefore, detecting the effects of competition between ostracods and Ae. camptorhynchus may be most sensitively measured through adult size rather than larval development and survival. On the other hand, it is possible that there is a coexistence between Ae. camptorhynchus and ostracods in these habitats and consequentially partitioning of resources driven by differences in species’ niches [45] and that the changes in adult size observed in these trials were driven by intra-specific competition, although further investigation is necessary.
Even though adult size was negatively affected by intra-specific resource limitation, males were significantly larger than females for the most limiting resource treatment. It is known that larval females take longer to develop as a trade-off for greater accumulation of resources which ultimately results in larger sizes and improved fecundity [48, 49]. However, under increased non-culicid exploitative resource limitation, there may be an effect on female Ae. camptorhynchus reproductive success. In fact, resource limitation is correlated with fewer oogenesis cycles resulting in reduced fecundity [50] and a decreased ability of mosquitoes to carry and transmit disease [8, 31, 41, 51]. Due to Ae. camptorhynchus being anautogenous [52] thereby requiring a blood meal to complete oogenesis, it is likely that this reduction in size for Ae. camptorhynchus limits vectorial abilities, although further research is necessary to understand the connection with Ae. camptorhynchus size and the ability to transmit RRV. A potential caveat is that experimental adults would not represent field adults, however, we have found substantial overlap in mean wing length between field caught adults and experimental adults (competition, 3.46 ±0.27, resources, 3.37 ± 0.26 and field, 3.60 ± 0.44).
Overall our experiments suggest exploitative competition between Ae. camptorhynchus and ostracods is limited in laboratory conditions. It is possible that environmental conditions may be more important than competition alone in that competitive effects may change given different abiotic conditions [2, 53, 54]. Such changes can be observed when habitats are drying out or with the addition of new invertebrates through rainfall or tide [55]. For example, hatching of first instar larvae may differ to ostracods hatching from dormancy [56, 57]. Such a situation could result in a time window in which first instar mosquitoes have a competitive advantage both in size and nutrient acquisition. It might also be possible that different life stages of Ae. camptorhynchus larvae are more sensitive to environmental changes or intra-specific competition [58]. Therefore, replicating these experiments in natural conditions in the field, with the addition of a fully crossed design (where all levels of resource limitation are also tested with ostracod densities against Ae. camptorhynchus development, survival and size) would benefit our understanding on the relationship between Ae. camptorhynchus inter- and intra-specific interactions and provide further insight on the complexity of competitive systems.
A limitation of our study is that we did not measure adult survival. Epidemiological models of mosquito-borne disease transmission incorporate adult longevity which can have significant effects on disease transmission [59]. However, environmental effects on larval development and survival have been demonstrated to be both condition- and species-specific, with strong associations between adult longevity, developmental times and body size demonstrated for some species [59, 60]. While resource limitation had a greater impact on Ae. camptorhynchus survival and development, it is possible that densities of non-culicid competitors (and resource limitation) may result in reduced adult longevity especially considering both treatments had an effect on adult size.
We demonstrate that intra-specific resource limitation and controphic competition have a direct impact on adult sizes, however, changes to Ae. camptorhynchus life history in exploitation competition environments is not so obvious. We conclude that controphic competition, although quite complex, may have a role in influencing vector-borne disease and implications to human health.
Supporting Information
(PDF)
Acknowledgments
We thank The Holsworth Wildlife Research Endowment–ANZ Trustees Foundation–and the Joan Woodberry Postgraduate Fellowship for providing funding support for this project. Senior Environmental Health Officer, Greg Robertson, at the Sorell Council granted authority to enter land and provided valuable site specific information. We also acknowledge the contribution of several volunteers that helped with all aspects of the field and laboratory work, namely Kelly Simpson and Jen Morgans.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by The Holsworth Wildlife Research Fund, ANZ Trustees Foundation http://www.eqt.com.au/not-for-profit-organisations/confirmed-2015-programs/holsworth-grants.aspx (RR), The Joan Woodberry Postgraduate Fellowship, University of Tasmania http://www.utas.edu.au/research/graduate-research/future-students/scholarships-and-fees (RR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blaustein L, Chase JM. Interactions between mosquito larvae and species that share the same trophic level. Annual Review of Entomology. 2007;52:489–507. [DOI] [PubMed] [Google Scholar]
- 2. Juliano SA. Species Interactions Among Larval Mosquitoes: Context Dependence Across Habitat Gradients. Annual Review of Entomology. 2009;54:37–56. 10.1146/annurev.ento.54.110807.090611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merritt RW, Dadd RH, Walker ED. Feeding behaviour, natural food, and nutritional relationships of larval mosquitos. Annual Review of Entomology. 1992;37:349–76. [DOI] [PubMed] [Google Scholar]
- 4. Knight TM, Chase JM, Goss CW, Knight JJ. Effects of interspecific competition, predation, and their interaction on survival and development time of immature Anopheles quadrimaculatus. Journal of Vector Ecology. 2004;29(2):277–84. [PubMed] [Google Scholar]
- 5. Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79(1):255–68. [Google Scholar]
- 6. Beketov MA, Liess M. Predation risk perception and food scarcity induce alterations of life-cycle traits of the mosquito Culex pipens . Ecological Entomology. 2007;32(4):405–10. [Google Scholar]
- 7. Werner AK, Goater S, Carver S, Robertson G, Allen GR. Environmental drivers of Ross River virus in southeastern Tasmania, Australia; towards strengthening public health interventions. Epidemiology and Infection. 2012;140(2):359–71. 10.1017/S0950268811000446 [DOI] [PubMed] [Google Scholar]
- 8. Nasci RS, Mitchell CJ. Larval diet, adult size, and susceptibility of Aedes aegypti (Diptera: Culicidae) to infection with Ross River virus. Journal of Medical Entomology. 1994;31(1):123–6. [DOI] [PubMed] [Google Scholar]
- 9. Mokany A, Shine R. Competition between tadpoles and mosquitoes: the effects of larval density and tadpole size. Australian Journal of Zoology. 2002;50(5):549–63. [Google Scholar]
- 10. Schneider JR, Chadee DD, Mori A, Romero-Severson J, Severson DW. Heritability and adaptive phenotypic plasticity of adult body size in the mosquito Aedes aegypti with implications for dengue vector competence. Infection, Genetics and Evolution. 2011;11(1):11–6. 10.1016/j.meegid.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eitam A, Blaustein L. Oviposition habitat selection by mosquitoes in response to predator (Notonecta maculata) density. Physiological Entomology. 2004;29(2):188–91. [Google Scholar]
- 12. Mokany A, Shine R. Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Austral Ecology. 2003;28(1):33–7. [Google Scholar]
- 13. Mokany A, Shine R. Competition between tadpoles and mosquito larvae. Oecologia. 2003;135(4):615–20. [DOI] [PubMed] [Google Scholar]
- 14. Duquesne S, Kroeger I, Kutyniok M, Liess M. The potential of cladocerans as controphic competitors of the mosquito Culex pipiens . Journal of Medical Entomology. 2011;48(3):554–60. [DOI] [PubMed] [Google Scholar]
- 15. Russell RC. Ross river virus; ecology and distribution. Annual Review of Entomology. 2002;47:1–31. [DOI] [PubMed] [Google Scholar]
- 16. Ballard JWO, Marshall ID. An investigation of the potential of Aedes camptorhynchus (Thom) as a vector of Ross River virus. Australian Journal of Experiemntal Biology and Medical Science. 1986;64:197–200. [DOI] [PubMed] [Google Scholar]
- 17. Aaskov J, Fokine A, Liu WJ. Ross River virus evolution: implications for vaccine development. Future Virol. 2012;7(2):173–8. [Google Scholar]
- 18. Carver S, Spafford H, Storey A, Weinstein P. The Roles of Predators, Competitors, and Secondary Salinization in Structuring Mosquito (Diptera: Culicidae) Assemblages in Ephemeral Water Bodies of the Wheatbelt of Western Australia. Environ Entomol. 2010;39(3):798–810. 10.1603/EN09235 [DOI] [PubMed] [Google Scholar]
- 19. Chapman HF, Hughes JM, Jennings C, Kay BH, Ritchie SA. Population structure and dispersal of the saltmarsh mosquito Aedes vigilax in Queensland, Australia. Medical and Veterinary Entomology. 1999;13(4):423–30. [DOI] [PubMed] [Google Scholar]
- 20. Van Schie C, Spafford H, Carver S, Weinstein P. Salinity tolerance of Aedes camptorhynchus (Diptera: Culicidae) from two regions in southwestern Australia. Australian Journal of Entomology. 2009;48(4):293–9. [Google Scholar]
- 21. Bader CA, Williams CR. Eggs of the Australian saltmarsh mosquito, Aedes camptorhynchus, survive for long periods and hatch in instalments: implications for biosecurity in New Zealand. Medical and Veterinary Entomology. 2011;25(1):70–6. 10.1111/j.1365-2915.2010.00908.x [DOI] [PubMed] [Google Scholar]
- 22. Carver S, Spafford H, Storey A, Weinstein P. Colonization of ephemeral water bodies in the wheatbelt of Western Australia be assemblages of mosquitoes (Diptera: Culicidae): Role of environmental factors, habitat and disturbance. Environ Entomol. 2009;38(6):1585–94. [DOI] [PubMed] [Google Scholar]
- 23. Williams DD. Temporary ponds and their invertebrate communities. Aquatic Conservation: Marine and Freshwater Ecosystems. 1997;7(2):105–17. [Google Scholar]
- 24. Rossi V, Benassi G, Belletti F, Menozzi P. Colonization, population dynamics, predatory behaviour and cannibalism in Heterocypris incongruens (Crustacea: Ostracoda). Journal of Limnology. 2011;70(1):102–8. [Google Scholar]
- 25. Walsh R. Australian Waterlife. Aquatic Micro-invertebrate Ecologist, Freshwater Ecology & Limnology ed: Available: www.australianwaterlife.com.au; 2015. [Google Scholar]
- 26. Carver S, Goater S, Allen GR, Parr R, Fearnley E, Weinstein P. Relationships of the Ross River Virus (Togoviridae: Alphavirus) vecor, Aedes camptorhynchus (Thomson) (Diptera:Culicidae), to biotic and abiotic factors in saltmarshes of south-eastern Tasmania, Australia: a preliminary study. Australian Journal of Entomology. 2011;50:344–55. [Google Scholar]
- 27.Rowbottom R, Carver S, Barmuta LA, Weinstein P, Allen GR. The abiotic/biotic predictor(s) that drive Aedes camptorhynchus Thomson (Diptera: Culicidae) populations in RRV regions of Tasmania. [Thesis]. In press unpublished data.
- 28. Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–52. 10.1038/nature09575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farjana T, Tuno N, Higa Y. Effects of temperature and diet on development and interspecies competition in Aedes aegypti and Aedes albopictus . Medical and Veterinary Entomology. 2012;26(2):210–7. 10.1111/j.1365-2915.2011.00971.x [DOI] [PubMed] [Google Scholar]
- 30. Tun-Lin W, Burkot TR, Kay BH. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Medical and Veterinary Entomology. 2000;14(1):31–7. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell-Foster K, Ma BO, Warsame-Ali S, Logan C, Rau ME, Lowenberger C. The influence of larval density, food stress, and parasitism on the bionomics of the dengue vector Aedes aegypti (Diptera: Culicidae): implications for integrated vector management. Journal of Vector Ecology. 2012;37(1):221–9. 10.1111/j.1948-7134.2012.00220.x [DOI] [PubMed] [Google Scholar]
- 32. Lounibos LP. Geographical and developmental components of adult size of neotropical Anopheles (Nyssorhynchus). Ecological Entomology. 1994;19(2):138–46. [Google Scholar]
- 33. Packer MJ, Corbet PS. Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae). Ecological Entomology. 1989;14(3):297–309. [Google Scholar]
- 34. Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchial Models: Cambridge University Press; 2006. [Google Scholar]
- 35.R. The R project for Statistical Computing. [cited 2014]; Available: http://www.R-project.org/.
- 36. Hadfield JD. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. Journal of Statistical Software. 2010;33(2):22. [Google Scholar]
- 37. Juliano SA. Population dynamics. Journal of the American Mosquito Control Association. 2007;23(2):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bevins SN. Timing of resource input and larval competition between invasive and native container-inhabiting mosquitoes (Diptera: Culicidae). Journal of Vector Ecology. 2007;32(2):252–62. [DOI] [PubMed] [Google Scholar]
- 39. Gilles JRL, Lees RS, Soliban SM, Benedict MQ. Density-Dependent Effects in Experimental Larval Populations of Anopheles arabiensis (Diptera: Culicidae) Can Be Negative, Neutral, or Overcompensatory Depending on Density and Diet Levels. Journal of Medical Entomology. 2011;48(2):296–304. [DOI] [PubMed] [Google Scholar]
- 40. Padmanabha H, Bolker B, Lord CC, Rubio C, Lounibos LP. Food Availability Alters the Effects of Larval Temperature on Aedes aegypti Growth. Journal of Medical Entomology. 2011;48(5):974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jennings CD, Kay BH. Dissemination barriers to Ross River virus in Aedes vigilax and the effects of larval nutrition on their expression. Medical and Veterinary Entomology. 1999;13(4):431–8. [DOI] [PubMed] [Google Scholar]
- 42. Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21(2):117–27. [Google Scholar]
- 43. Knight J. A Model of Mosquito–Mangrove Basin Ecosystems with Implications for Management. Ecosystems. 2011;14(8):1382–95. [Google Scholar]
- 44. Stav G, Blaustein L, Margalit Y. Individual and interactive effects of a predator and controphic species on mosquito populations. Ecological Applications. 2005;15(2):587–98. [Google Scholar]
- 45. Amarasekare P. Competitive coexistence in spatially structured environments: a synthesis. Ecology Letters. 2003;6(12):1109–22. [Google Scholar]
- 46. Morin PJ. Community ecology / Peter J. Morin: Malden, Mass.; Oxford: Blackwell Science; 1999. [Google Scholar]
- 47. Daugherty MP, Juliano SA. Leaf Scraping Beetle Feces Are a Food Resource for Tree Hole Mosquito Larvae. American Midland Naturalist. 2003;150(1):181–4. [Google Scholar]
- 48. Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus . Journal of Vector Ecology. 2000;25:212–7. [PubMed] [Google Scholar]
- 49. Clements AN. The Biology of Mosquitoes, Volume 1 New York: Chapman and Hall; 1992. [Google Scholar]
- 50. Telang A, Li Y, Noriega FG, Brown MR. Effects of larval nutrition on the endocrinology of mosquito egg development. Journal of Experimental Biology. 2006;209(4):645–55. [DOI] [PubMed] [Google Scholar]
- 51. Muturi EJ, Kim C-H, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Tropical Medicine & International Health. 2011;16(8):955–64. [DOI] [PubMed] [Google Scholar]
- 52. Barton PS, Aberton JG. Larval development and autogeny in Ochlerotatus camptorhynchus (Thomson) (Diptera: Culicidae) from southern Victoria. Proceedings of the Linnean Society of New South Wales. 2005;126(1):261–7. [Google Scholar]
- 53. Tilman D. Resource competition and community structure / David Tilman: Princeton, N.J. Princeton University Press, 1982; 1982. [Google Scholar]
- 54. Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: The role of noncompeting life-history stages. Ecology. 2005;86(12):3289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chase JM, Knight TM. Drought-induced mosquito outbreaks in wetlands. Ecology Letters. 2003;6:1017–24. [Google Scholar]
- 56. Khatchikian CE, Dennehy JJ, Vitek CJ, Livdahl TP. Environmental effects on bet hedging in Aedes mosquito egg hatch. Evol Ecol. 2010;24(5):1159–69. [Google Scholar]
- 57. Campos RE, Sy VE. Variation in the hatching response of Ochlerotatus albifasciatus egg batches (Diptera: Culicidae) in temperate Argentina. Memorias Do Instituto Oswaldo Cruz. 2006;101(1):47–53. [DOI] [PubMed] [Google Scholar]
- 58. Silberbush A, Tsurim I, Rosen R, Margalith Y, Ovadia O. Species-Specific Non-Physical Interference Competition among Mosquito Larvae. PLoS ONE. 2014;9(2):e88650 10.1371/journal.pone.0088650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus . Medical and Veterinary Entomology. 2009;23(1):62–8. 10.1111/j.1365-2915.2008.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mogi M, Miyagi I, Abadi K, Syafruddin. Inter- and Intraspecific Variation in Resistance to Desiccation by Adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. Journal of Medical Entomology. 1996;33(1):53–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.