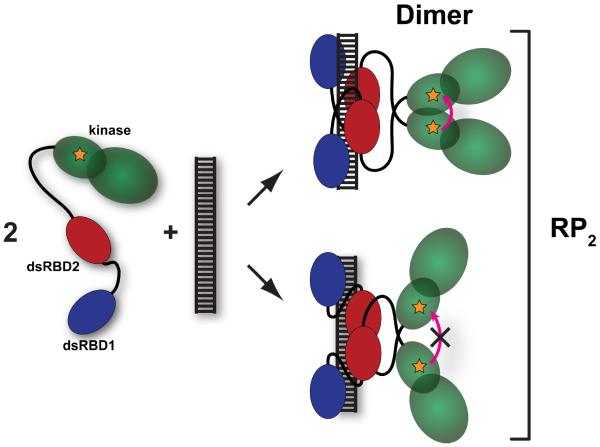
Figure 4. Schematic of formation of the kinase domain dimer.
The kinase domain is depicted in green, dsRBD1 in blue and dsRBD2 in red. The fluorophore is depicted as an orange star. Upon binding of two pAz-F261-A488 monomers to a single dsRNA the kinase domains may dimerize in the back-to-back parallel configuration, leading to homo-FRET (top). Alternatively, the protein may bind to the dsRNA in a configuration that does not support kinase domain dimerization that leads to homo-FRET (bottom). The term RP2 encompasses all forms of the RNA containing two bound PKR monomers.