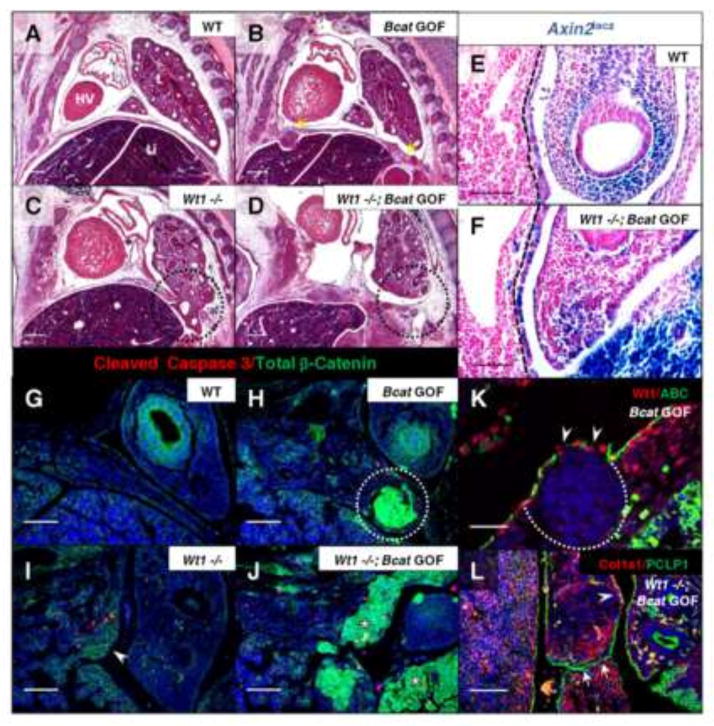
Fig. 4.
Induction of constitutively active β-catenin in the Wt1 lineage results in mesenchymal overgrowth sufficient to close Wt1 null diaphragm defects. H&E stained sagittal sections of Wt1CreERT2;Bcatex3fx embryos, injected with tamoxifen at E10.5 and harvested at E13.5 (A–D), illustrate phenotypes of single and double mutants compared to wildtype. Yellow arrows indicate where nodules have formed in the gain-of-function diaphragm (B) and the affected region of the posterior diaphragm in the Wt1 single and double mutants is circled (C, D). Wt1CreERT2;Bcatex3fx;Axin2lacz embryos at E11.5 were whole-mount X-gal stained and sectioned sagittally (E, F). The positively stained posterior diaphragm mesothelium is demarcated by dotted lines. Co-immunofluorescence of Total β-catenin and Cleaved Caspase 3 was carried out on Wt1CreERT2;Bcatex3fx E12.5 paraffin sections (G–J). Constitutively active β-catenin is shown in nodules (circle, H) and adjacent to Wt1 null diaphragm defects (asterisks, J). Presence of only membranous total β-catenin is indicated in single Wt1 null (arrowhead, I). Co-immunofluorescence was carried out at E13.5 of Wt1/Active β-catenin in diaphragm nodules in β-catenin gain-of-function (K) and Collagen1a1/Podocalyxin (PCLP1) in Wt1CreERT2;Bcatex3fx double mutant (L) posterior diaphragms. Dotted line outlines the nuclei-dense growth and arrowheads indicate loss of phosphorylation site-specific antibody staining where β-catenin exon 3 has been excised (K). Arrows indicate mesothelial fusion (L), where diaphragm tissue has grown together after the initial Wt1 defect was already formed. Arrowhead labels a region of wildtype localization of Col1a1. Abbreviations: HV, Heart Ventricle; L, lung; Li, liver. Scale bars: A–D, 200 μm; E–J, and L, 100 μm; K, 50 μm.