FIGURE 1.
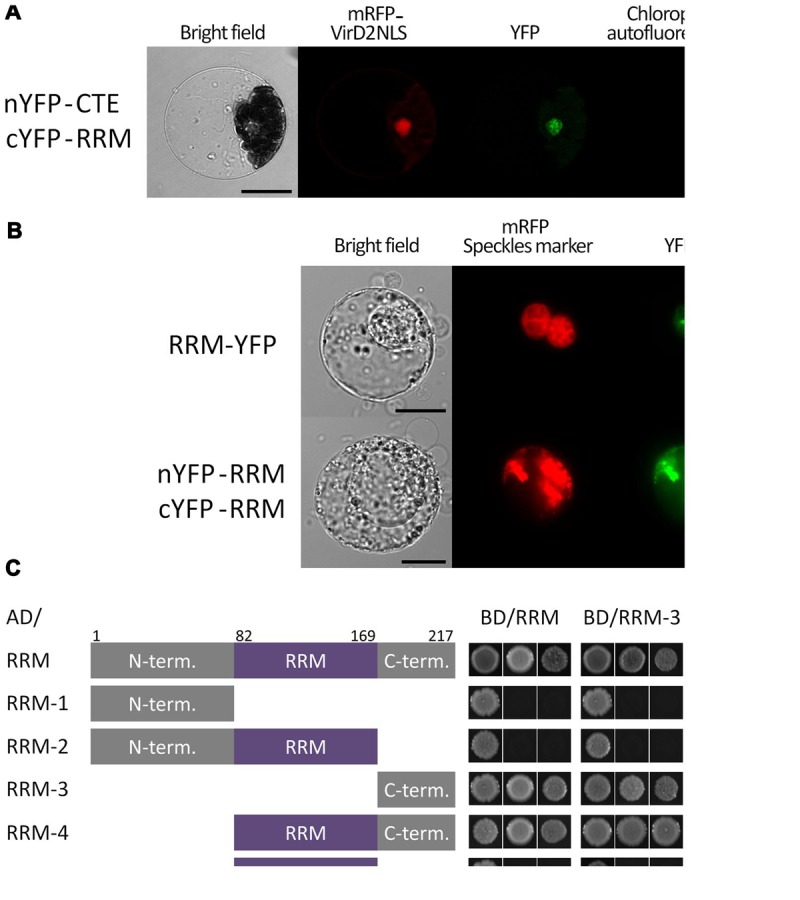
Subcellular localization and dimerization of the RRM protein and verification of its interaction with the CTE domain of AtTERT. (A) BiFC in Arabidopsis leaf protoplasts confirmed a nuclear interaction of the RRM protein with AtTERT CTE domain. Protoplasts were co-transfected with plasmids encoding nYFP-tagged TERT(CTE2), cYFP-tagged RRM, and mRFP-VirD2(NLS) to label cell nuclei; nYFP- and cYFP-GAUT10 constructs served as negative control (not shown). YFP fluorescence is shown in green, mRFP fluorescence in red, and chlorophyll autofluorescence in blue pseudocolor. Scale bar indicates 10 μm. (B) RRM-YFP co-localizes with a pSRp30-RFP nuclear speckle marker in tobacco BY-2 protoplasts. The same localization pattern was observed for interaction of nYFP-RRM with cYFP-RRM. YFP fluorescence is shown in green, and mRFP fluorescence of the VirD2(NLS) marker is shown in red. Scale bars indicate 20 μm. (C) A Y2H system was used to assess RRM dimerization. Two sets of plasmids carrying full-length RRM or indicated RRM segments fused to either the GAL4 DNA-binding domain (BD) or the GAL4 activation domain (AD) were constructed and introduced into Saccharomyces cerevisiae PJ69-4a carrying His3 and Ade2 reporter genes. Co-transformation with an empty vector served as a negative control (not shown). Full-length RRM protein self-interacted on both histidine and stringent adenine selection plates. The same result was observed for interactions of the RRM-4(82–217) and the RRM-3(170–217) fragments with the full-length RRM protein, each other, and themselves, suggesting that the RRM-protein C-terminus is responsible for protein homodimerization. None of the other fragments [RRM-1(1–81), RRM-2(1–169), and RRM-5(82–169)] showed interaction, although their successful expression was confirmed by immunoblotting (Supplementary Figure S1).
