FIGURE 2.
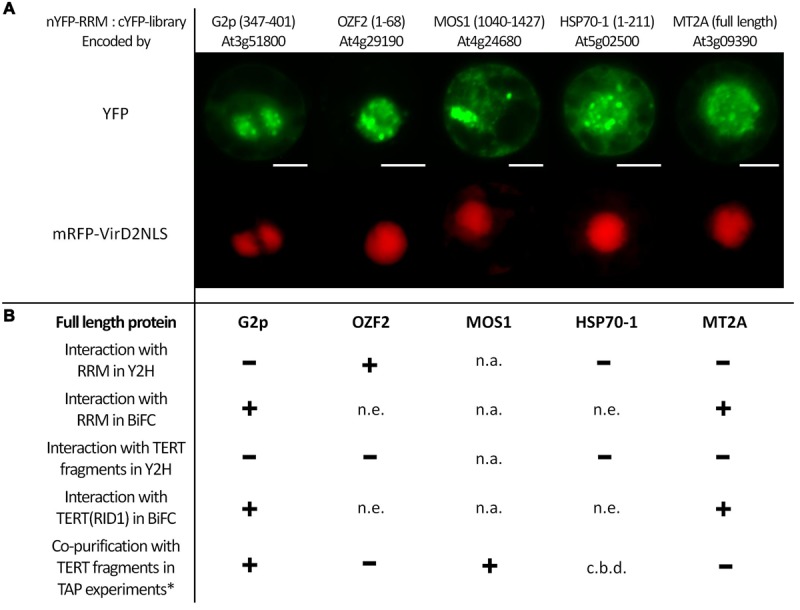
BiFC screening of an Arabidopsis cDNA library identified interaction partners of the RRM protein. (A) Interactions of nYFP-RRM with cYFP-tagged protein fragments of G2p(347–401), OZF2(1–68), MOS1(1040–1427), HSP70-1(1–211) and full-length MT2A protein identified by screening a cYFP-tagged cDNA library. Tobacco BY-2 protoplasts were co-transfected with plasmids encoding a mRFP-VirD2NLS nuclear marker, nYFP-RRM, and one of the five interacting cYFP-tagged proteins. YFP fluorescence is shown in green, and mRFP fluorescence is shown in red. Scale bars indicate 20 μm. (B) Summary of investigated protein–protein interactions of full-length G2p, OZF2, HSP70-1, and MT2A proteins with full-length RRM protein and TERT fragments. One of the fragments (RID1) was used in BiFC; all other TERT fragments were investigated using a Y2H system. Using the GAL4-based Y2H system in S. cerevisiae PJ69-4a carrying His3 and Ade2 reporter genes, we confirmed interaction only between OZF2-AD and RRM-BD on both histidine and stringent adenine selection plates. Other investigated combinations were negative, excluding the OZF2-BD construct that showed false positive interactions, and the HSP70-1-AD construct that was not expressed. Protein expression was checked by immunoblotting (Supplementary Figure S1). In addition to interaction of MT2A with full-length RRM protein shown on (A), BiFC analysis in tobacco BY-2 protoplasts revealed positive interactions of MT2A with the TERT(RID1) fragment and also of full-length G2p protein with both full-length RRM protein, and the TERT(RID1) fragment (Supplementary Figure S2). n.a., not analyzed, n.e., not expressed, c.b.d., cannot be determined. ∗G2p and MOS1 co-purified with TERT fragments in other work of our group (Majerska et al., manuscript in preparation).
