FIGURE 5.
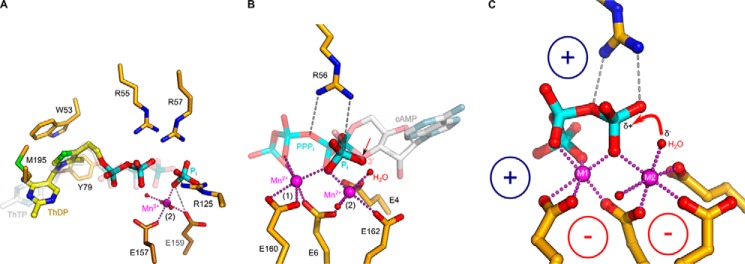
TTM proteins use a two-metal catalytic mechanism. A, close-up view of the mouse ThTPase tunnel center with either the ThTP substrate bound (transparent gray) or the ThDP/Pi products post-catalysis (in yellow, in bond representation). The thiamine part of ThTP is buried in a pocket close to the C-terminal plug helix of the TTM domain formed by Tyr-79 and Met-195; the thiazole ring makes a stacking interaction with Trp-53 (in yellow, in bond representation). The product Pi is coordinated by a Mn2+ ion bound to site 2 and by Arg-125. B, close-up of the ygiF active site (in yellow, in bond representation) bound to PPPi and two Mn2+ ions (magenta spheres) in sites 1 and 2. The Mn2+ ion in site 2 coordinates a water molecule (red sphere), which is well positioned to act as nucleophile. Structural superposition with a product-bound class IV adenylate cyclase (PDB ID 3N10) reveals the O3′ of cAMP in the same position as the water molecule in ygiF. This position is also occupied by an oxygen atom of the product Pi located in the AtTTM3 post-catalysis complex. C, the suggested mechanism for acidic-patch containing TTM proteins. The metal ion in site 1 coordinates the triphosphate moiety of the substrate to the tunnel center by interacting with a conserved Glu residue. Three additional glutamates form metal binding site 2, which coordinates and polarizes a water molecule to attack the γ-phosphate of the substrate. Conserved basic residues in the tunnel center are involved in substrate binding and potentially stabilize the pyrophosphate leaving group.