Abstract
High–oleic acid soybean oil (H-OSBO) is a trait-enhanced vegetable oil containing >70% oleic acid. Developed as an alternative for trans-FA (TFA)-containing vegetable oils, H-OSBO is predicted to replace large amounts of soybean oil in the US diet. However, there is little evidence concerning the effects of H-OSBO on coronary heart disease (CHD)6 risk factors and CHD risk. We examined and quantified the effects of substituting high-oleic acid (HO) oils for fats and oils rich in saturated FAs (SFAs), TFAs, or n–6 (ω-6) polyunsaturated FAs (PUFAs) on blood lipids in controlled clinical trials. Searches of online databases through June 2014 were used to select studies that defined subject characteristics; described control and intervention diets; substituted HO oils compositionally similar to H-OSBO (i.e., ≥70% oleic acid) for equivalent amounts of oils high in SFAs, TFAs, or n–6 PUFAs for ≥3 wk; and reported changes in blood lipids. Studies that replaced saturated fats or oils with HO oils showed significant reductions in total cholesterol (TC), LDL cholesterol, and apolipoprotein B (apoB) (P < 0.05; mean percentage of change: −8.0%, −10.9%, −7.9%, respectively), whereas most showed no changes in HDL cholesterol, triglycerides (TGs), the ratio of TC to HDL cholesterol (TC:HDL cholesterol), and apolipoprotein A-1 (apoA-1). Replacing TFA-containing oil sources with HO oils showed significant reductions in TC, LDL cholesterol, apoB, TGs, TC:HDL cholesterol and increased HDL cholesterol and apoA-1 (mean percentage of change: −5.7%, −9.2%, −7.3%, −11.7%, −12.1%, 5.6%, 3.7%, respectively; P < 0.05). In most studies that replaced oils high in n–6 PUFAs with equivalent amounts of HO oils, TC, LDL cholesterol, TGs, HDL cholesterol, apoA-1, and TC:HDL cholesterol did not change. These findings suggest that replacing fats and oils high in SFAs or TFAs with either H-OSBO or oils high in n–6 PUFAs would have favorable and comparable effects on plasma lipid risk factors and overall CHD risk.
Keywords: oleic acid, high–oleic acid soybean oil, monounsaturated fatty acids, polyunsaturated fatty acids, cardiovascular disease, fatty acids, lipids, randomized controlled trials
Introduction
The role of edible fats and oils in health and disease continues to evolve as further knowledge is gained about the relation between dietary fats and FAs and chronic diseases, particularly coronary heart disease (CHD). For instance, in response to early public health recommendations to move away from animal fats and tropical oils to reduce CHD risk, the food industry adopted the use of partially hydrogenated vegetable oils (PHVOs) to achieve the same functional characteristics provided by animal fats in food products (1). PHVOs and the trans-FA (TFA) components produced by partial hydrogenation of liquid vegetable oils appeared to be a suitable alternative because of their ability to replace animal fats high in saturated fat while providing adequate functionality for a wide range of product applications, increased flavor stability and shelf life, resistance to oxidation, lower cost, and consistent availability. However, on the basis of evidence from numerous clinical and prospective cohort studies it is now recognized that the consumption of TFAs, in amounts achievable in Western diets (2), adversely affects CHD risk factors and is associated with a higher risk of CHD (3–5). Current US government dietary guidelines recommend keeping TFA consumption as low as possible by limiting the intake of foods that contain TFAs and by limiting other solid fats (6). In addition, since 2006, food manufacturers are required to state the quantity of TFAs per serving on the Nutrition Facts panel of all packaged food labels. As a result, many food products were reformulated to contain lower amounts of TFAs and have led to substantial reductions in TFA intake (2, 7).
A number of ingredient alternatives are available to replace PHVOs in oil-based food products. In general, the preponderance of edible fats and oils are used in 3 areas of food product applications: salad and cooking oils, solid fats (e.g., margarines, shortenings), and frying oils. Salad and cooking oils is the largest category, accounting for ∼65% of the total fats and oils consumed in the United States (8). The need for TFA alternatives in this category is low, because most of these products contain little or no TFAs and typically include oils rich in PUFAs and MUFAs such as soybean oil (SBO), corn oil, canola oil, and others (9). In contrast, solid-fat products that contain PHVOs such as margarines and shortenings present greater product challenges, including management of functional properties such as melting point, lubricity, moisture barrier, and creaming ability to produce a product low in TFAs with parity performance to its PHVO counterpart. To minimize TFAs while meeting functional and stability requirements many of the current approaches have typically used oil blends that contain small proportions of saturated fats high in palmitic acid (e.g., palm, palm fractions) and a high proportion of high–oleic acid (HO) oils or oils moderately high in linoleic acid (LA; 18:2n–6) and α-linolenic acid (ALA; 18:3n–3) (2). Frying fats and shortenings used in restaurants for preparing deep-fried foods and for commercial frying of packaged foods such as snacking chips represent a large source of PHVOs and are known to contain relatively high amounts of TFAs (2). Alternative oils with increased amounts of oleic acid (i.e., ∼50–65%), decreased amounts of LA (i.e., ∼20–30%), and low amounts of ALA (∼<3%) have proven effective replacements for PHVOs because they are able to withstand the high temperatures of commercial frying, provide relatively long fry life, and are resistant to producing off flavors because of thermal deterioration and oxidative processes (1). The replacement of PHVO frying fats with alternative oils was examined in a FA analysis of 327 fast-food items by Health Canada which suggested that products achieving a goal of ≤5% TFAs was accomplished by using predominantly HO vegetable oils (2). Examples of HO oils (i.e., >70% oleic acid) include olive oil, HO sunflower oil, HO safflower oil, and newer trait-enhanced oils such as HO canola oil and H-OSBO (Table 1).
TABLE 1.
FA composition of representative conventional vegetable oils and H-OSBO1
SBO is the most consumed edible oil in the United States because of its availability, cost, and versatility in food product applications. In 2004, of the ∼12.3 billion kg of edible oils used in the United States, SBO accounted for ∼7.9 billion kg (i.e., ∼67%) (8). SBO is high in LA and ALA. Hence, for use in baking and frying, shortenings in which high heat stability is required, SBO was traditionally partially hydrogenated to produce stable solid or semisolid fats. In 2006 an estimated 41% of the total SBO used in edible products was partially hydrogenated (1), accounting for nearly 82% of the 7.5 billion pounds of baking and frying shortenings produced in the United States and considerable amounts used in production of margarines particularly hard-stick margarines (8). However, as a result of US and Canadian regulatory requirements for mandatory labeling of trans fats on packaged foods and mounting consumer concerns about the health effects of TFAs, the food industry has been reducing or eliminating TFAs in its products (1, 13, 14). Between 2007 and 2011, 66% of identified brand-name products in US supermarkets that contained ≥0.5 g TFAs per serving had reduced TFA content to <0.5 g per serving (15). Although SBO continues to be the dominant edible oil in the US market, in 2011–2012 it accounted for 50% of the disappearance of all edible fats and oils used in the United States, down from 65% a decade earlier (16). The market trend away from SBO and SBO-derived hydrogenated ingredients has made development and expanded production of H-OSBO a compelling alternative because of its high heat and oxidative stability for use in both high temperature and ambient temperature food applications, and because of potential cost advantages due to the economy of scale of US soybean production.
Because H-OSBO requires no hydrogenation to be used in many processed food applications, including deep frying and baking, the use of H-OSBO in processed food products provides opportunities for consumers to reduce their intake of TFAs. Large-scale substitution of SBO with H-OSBO could affect the intake of other FAs, including the essential FA, ALA. The ALA content of H-OSBO is ∼2.5–3.0% compared with ∼7% for SBO. However, SBO is hydrogenated for use in many food applications which reduces its ALA content. Therefore, the FA content of foods produced from H-OSBO and partially hydrogenated SBO (PHSBO) will differ primarily in the amounts of TFAs and not ALA content. In a study that estimated the impact of substituting low ALA-SBO, a SBO variety that contains only 2.4% ALA, for PHSBO in several food categories, dietary TFA intake was reduced by 45%, whereas no reduction was found in ALA intake (17). Because the levels of ALA in low ALA-SBO and H-OSBO are similar, the dietary effect of substituting H-OSBO for PHSBO on ALA intake would be expected to be similar to that estimated for low ALA-SBO (17).
In the only randomized controlled trial (RCT), to our knowledge, that compared the effects on blood lipids of a diet high in H-OSBO with a diet with an isocaloric amount of SBO (high PUFAs), no substantial differences were observed in any of the lipid biomarkers, suggesting that MUFAs and PUFAs are interchangeable for their effects on blood lipids. Both diets were effective in substantially lowering LDL cholesterol, apoB, and the ratio of total cholesterol (TC) to HDL cholesterol (TC:HDL cholesterol) compared with a diet that contained TFAs from PHSBO (18). Previous reviews that assessed the clinical evidence on the effects of MUFAs on traditional cardiovascular disease (CVD) risk factors concluded that the MUFA class of FAs favorably affects plasma lipids and lipoproteins when substituting for SFAs or carbohydrate-containing diets (19, 20). In a meta-analysis of 13 RCTs that estimated the change in CHD risk when TFA-containing diets are substituted with unsaturated FA-containing vegetable oils, replacing 7.5% of energy from PHVOs with an equivalent amount of HO- or PUFA-containing vegetable oils resulted in risk reductions of 9.2% and 8.8%, respectively (21). Although these studies indicate that replacing saturated fats or PHVOs with high MUFA-containing vegetable oils has a favorable effect on CHD risk, there is a need for additional clarity. Given the predicted increased presence of H-OSBO in the US diet, there is a need to understand the quantitative and qualitative impact of HO oils on CVD risk compared with other fats and oils in the diet. The purpose of this review is to provide insight into potential CVD health effects of H-OSBO by assessing the available clinical evidence on the direct isocaloric substitution of HO oils compositionally similar to H-OSBO (i.e., >70% oleic acid) for other fats and oils. Specifically, the evidence is summarized into 3 separate but related areas of substituting HO oils for fats and oils high in 1) SFAs, 2) TFAs, and 3) n–6 PUFAs (primarily LA).
Methods
We searched for all controlled dietary trials that examined the effect of substituting single or blends of HO vegetable oils compositionally similar to H-OSBO (i.e., >70% oleic acid) for isocaloric or near isocaloric amounts of fats and oils high in SFAs, TFAs, or PUFAs on plasma lipids and lipoproteins in normal and hypercholesterolemic adults aged >19 y. Studies that were ≥3 wk in duration were included for assurance of plasma lipid and lipoprotein stabilization after dietary changes (22). Those studies that provided sufficient data to calculate the percentage of energy contributed by the experimental fats and oils in the intervention diets and sufficient data to calculate the percentage of change in plasma lipids and lipoproteins resulting from the substitution of HO oils for the comparison fats and oils were included. Controlled dietary trials that consisted of randomized crossover, randomized parallel and, in some cases, nonrandomized sequential crossover designs were included. Studies were excluded if 1) oils contained <70% oleic acid, 2) did not report the macronutrient and FA composition of the experimental diets, 3) only reported TC results, 4) compared HO oils with combinations of SFA, TFA, and PUFA test fats or to test fats that were mainly n–3 PUFAs, and 5) compared HO oil outcomes only with baseline diets. Results from studies in which the test fats were not administered as part of a mixed-food diet (e.g., liquid diets) are acknowledged and discussed but were not included in our analyses because the intake of nutrients is atypical and to avoid reporting potentially skewed plasma lipid and lipoprotein results.
Search strategy
Searches were conducted for articles published through June 2014 and were limited to literature in the English language, human adults aged >19 y, and those clinical trials found using PubMed, Google Scholar, Cochrane Library database, and hand-searching of reference lists from reviews and meta-analyses. When necessary, authors and other experts were contacted to clarify methods or results. Search terms and keywords included but were not limited to “lipids,” OR “fats,” OR “oils,” OR “fatty acids,” OR “saturated fatty acids,” OR “saturated fats,” OR “trans fatty acids,” OR “trans fat,” OR “unsaturated fatty acids,” OR “unsaturated fats” OR “polyunsaturated fatty acids,” OR “polyunsaturated fats,” OR “omega-6,” OR “linoleic acid,” OR “linoleate,” OR “monounsaturated fatty acids,” OR “monounsaturated fats,” OR “oleic acid,” OR “oleate,” OR “high oleic,” OR “butter,” OR “milk fat,” OR “lard,” OR “lard,” OR “palm oil,” OR “palm olein,” OR “partially hydrogenated vegetable oil,” OR “hydrogenated vegetable oils,” OR “soybean oil,” OR “sunflower oil,” OR “corn oil,” OR “safflower oil,” OR “high oleic sunflower oil,” OR “high oleic safflower oil,” OR “high oleic canola oil,” OR “high oleic rapeseed oil,” OR “high oleic soybean oil,” OR “olive oil,” AND “heart disease,” OR “coronary heart disease,” OR, “cardiovascular disease,” AND “serum” OR “plasma,” OR “blood,” OR “hyperlipidemic,” OR “normolipidemic,” OR “hypercholesterolemic,” OR “normocholesterolemic,” OR “healthy,” OR “lipoproteins,” OR “cholesterol,” OR “low-density lipoprotein cholesterol,” OR “LDL,” OR “high-density lipoprotein cholesterol,” OR “HDL,” OR “triglycerides,” OR “triacylglycerol,” OR “apolipoproteins,” OR “apolipoprotein B,” OR “apoB,” OR “apolipoprotein A-1,” OR “apoA-1.”
Study selection process
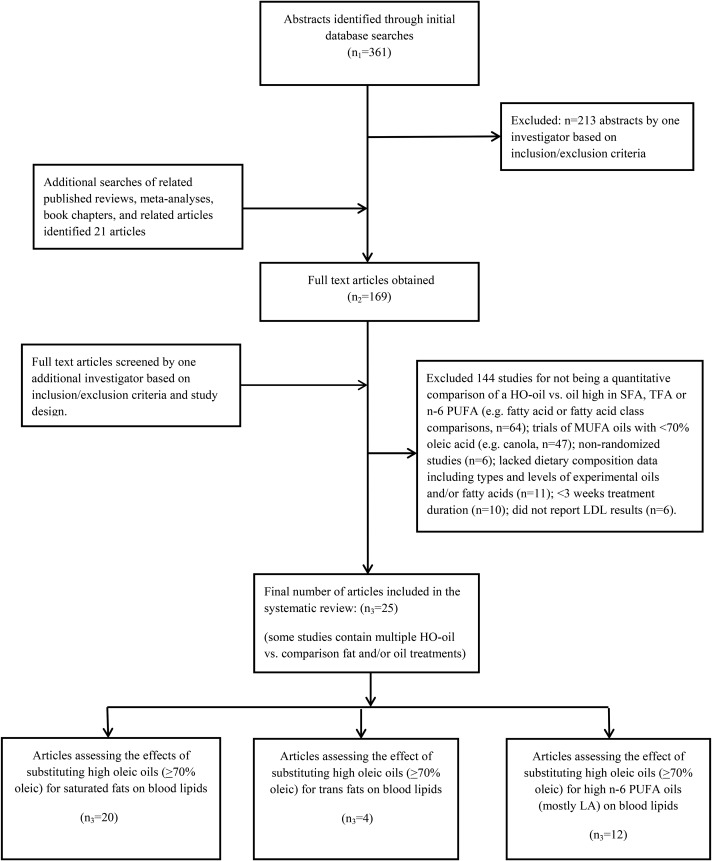
A total of 361 abstracts were identified, and 213 were excluded after reviewing titles and abstracts (Figure 1). After additional searches, full texts of 169 articles were obtained and examined to determine that inclusion/exclusion criteria were met for inclusion and the removal of duplicate publications. Overall, the final number of published studies that examined the plasma lipid and lipoprotein effects of substituting HO oils for fats high in SFAs, TFAs, and n–6 PUFAs was 20, 4, and 11, and consisted of 23, 6, and 11 dietary comparisons, respectively. In some instances, studies contained dietary comparisons of HO oils with multiple fats and oils relevant to this review.
FIGURE 1.
PRISMA flow chart of search strategy and study selection process. HO, high-oleic acid; LA, linoleic acid; TFA, trans FA.
Data extraction
Data were extracted from each of the final identified studies, including year of publication, subject characteristics, type of design and duration, control and intervention diet macronutrient composition, type and amount of control and intervention oils, and plasma TC, LDL cholesterol, HDL cholesterol, TGs, TC:HDL cholesterol, and apoB and apoA-1 results.
Outcome measures
For the individual studies included in these analyses we calculated the percentage of change in the following blood lipid components: TC, LDL cholesterol, HDL cholesterol, TGs, apoB, and apoA-1, resulting from substituting HO oils for oils high in SFAs, TFAs, or n–6 PUFAs as follows: blood lipid component (%Δ) = (HO oil treatment) − (substituted oil)/(substituted oil) × 100%.
For determining the average percentage of change of each blood lipid component from all studies that substituted HO oils for oils high in SFAs, TFAs, or n–6 PUFAs we calculated the weighted mean change (WMC) percentage that was weighted on the basis of study subject number as follows:
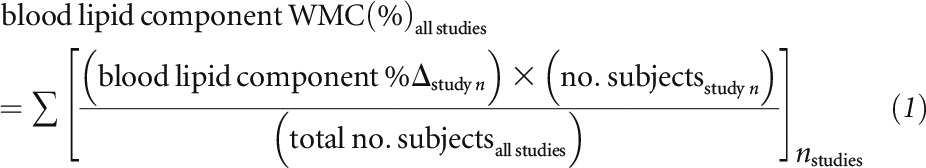 |
Results
Composition of HO oils
Table 1 summarizes the FA composition of vegetable oils relevant to this review. Vegetable oils shown in Table 1 which contain oleic acid amounts similar to H-OSBO (i.e., >70% oleic acid) and whose effects on blood lipid were compared in clinical trials with fats and oils high in SFAs, TFAs, and PUFAs include olive oil, HO sunflower oil, HO safflower oil, and HO canola oil. The oleic acid amounts of these HO comparison oils ranged from 71% to 79% of total FAs compared with 72% and 75% for the 2 varieties of H-OSBO available in the marketplace. Amounts of SFA, LA, and ALA in the HO comparison oils ranged as follows: 6–16%, 10–17%, and 0.1–2%, respectively, compared with 7% and 10.5%; 16%; and 7%, 3%, and 2.5%, respectively, for the 2 H-OSBO varieties.
Effects of substituting HO oils for fats high in SFAs or TFAs on plasma lipids and lipoproteins
In this section, 21 studies that consisted of 30 diet comparisons that primarily assessed the blood lipid effects of substituting dietary MUFAs from HO oils (i.e., >70% oleic acid) for isocaloric amounts of fats and oils high in SFAs or TFAs were reviewed (Table 2).
TABLE 2.
Controlled clinical trials that assessed the substitution of HO oils (>70% oleic acid) for saturated fats and trans fats on plasma lipids and lipoproteins1
| Author, year, (reference) | Design (type of diet) | Subjects/baseline blood lipids, mmol/L | Duration of diet, d | HO diet (en% from test fat) [diet en%] | Control diet (en%: from test fat) [diet en%] | TC, %Δ | LDL-C, %Δ | HDL-C, %Δ | TGs, %Δ | TC:HDL-C, %Δ | apoB, %Δ | apoA-1, %Δ |
| Substituting HO oils for saturated fats | ||||||||||||
| Mattson and Grundy, 1985 (23) | RC (liquid diet) | US adults, 47–69 y, HC, n = 20; | 28 | (HO safflower oil: 40 en%) | (Palm oil: 40 en%) | ↓12.0a | ↓16.8a | ↓2.5 | ↓3.8 | NR | NR | NR |
| TC: 6.80 | TF: [40 en%] | TF: [40 en%] | ||||||||||
| LDL-C: NR | SFAs: 3.4 | SFAs: 20.0 | ||||||||||
| HDL-C: NR | MUFAs: 29.0 | MUFAs: 16.0 | ||||||||||
| TGs: NR | PUFAs: 7.0 | PUFAs: 4.0 | ||||||||||
| Bonanome and Grundy, 1988 (24) | RC (liquid diet) | US adults, mean age 64 y, HC, n = 11; | 21 | (HO safflower oil: 40 en%) | (Palm oil: 40 en%) | ↓10.3b | ↓15.2b | ↑3.7 | ↓4.8 | NR | NR | NR |
| TC: 5.87 | TF: [40 en%] | TF: [40 en%] | ||||||||||
| LDL-C: 3.98 | SFAs: 3.2 | SFAs: 20.0 | ||||||||||
| HDL-C: 1.11 | MUFAs: 32.0 | MUFAs: 16.0 | ||||||||||
| TGs: 1.64 | PUFAs: 5.0 | PUFAs: 4.0 | ||||||||||
| Mensink and Katan, 1990 (25) | RC (mixed-food diet) | Dutch men and women, mean age 26 y, NC, n = 59; | 23 | (HO sunflower + olive oil + rapeseed oils: 9.9 en%) | (Palm oil +palm kernel: 15.1 en%) | ↓10.8c | ↓14.9c | 0.0 | ↓13.8c | ↓10.0c | ↓10.0c | ↓0.67 |
| TC: 4.75 | TF: [39.6 en%] | TF: [39.0 en%] | ||||||||||
| LDL-C: 2.18 | SFAs: 9.5 | SFAs: 19.4 | ||||||||||
| HDL-C: 2.38 | MUFAs: 24 | MUFAs: 14.7 | ||||||||||
| TGs: 0.96 | PUFAs: 5.0 | PUFAs: 3.4 | ||||||||||
| Wardlaw and Snook, 1990 (26) | Unbalanced RC (mixed-food diet) | US men, mean age 34.7 y, HC, n = 20; | 35 | (HO sunflower oil: 34.0 en%) | (Butter: 34.8 en%) | ↓16.5d | ↓20.9d | 0.0 | ↓9.6 | NR | ↓22.5d | ↓4.5 |
| TC: 5.78 | TF: [41 en%] | TF: [40 en%] | ||||||||||
| LDL-C: 3.93 | SFAs: 7.0 | SFAs: 21 | ||||||||||
| HDL-C: 1.09 | MUFAs: 28 | MUFAs:14 | ||||||||||
| TGs: 1.95 | PUFAs: 6.0 | PUFAs: 5 | ||||||||||
| Denke and Grundy, 1992 (27) | RC (liquid diet) | US men, mean age 63 y, NC, n = 14; | 21 | (HO sunflower oil: 40 en%) | (Palm oil: 40 en%) | ↓6.9a | ↓15.8a | ↓7.8 | ↓0.9 | NR | NR | NR |
| TC: NR | TF: [40 en%] | TF: [40 en%] | ||||||||||
| LDL-C: NR | SFAs: 2.8 | SFAs: 19.6 | ||||||||||
| HDL-C: NR | MUFAs: 30.0 | MUFAs: 16.0 | ||||||||||
| TGs: NR | PUFAs: 6.2 | PUFAs: 4.0 | ||||||||||
| Mata et al., 1992 (28) | NRan, SC (mixed-food diet) | Spanish women, mean age 43 y, NC, n = 21; | 42 | (Olive oil: 19.5 en%) | (Palm oil + butter: 19.3 en%) | ↓8.9d | ↓18.6d | ↑5.6d | ↑9.5a | NR | ↓14.4h | ↑6.8d |
| TC: 4.94 | TF: [35.4 en%] | TF: [ 35.0] en% | ||||||||||
| LDL-C: 2.92 | SFAs: 10.3 | SFAs: 18.0 | ||||||||||
| HDL-C: 1.55 | MUFAs: 21.4 | MUFAs: 13.5 | ||||||||||
| TGs: 0.96 | PUFAs: 3.3 | PUFAs: 3.2 | ||||||||||
| Ng et al., 1992 (29) | RC (mixed-food diet) | Malaysian men and women, 21–41 y, HC, n = 33; | 42 | (Olive oil: 22.6 en%) | 1. (Palm olein: 22.6 en%) | ↑1.0 (M) | ↓0.7 (M) | 0.0 (M) | ↑0.8 M, | NR | NR | NR |
| 0.0 F | ↑1.6 F | 0.0 F | ↓8.4 F | NR | NR | NR | ||||||
| TC: 4.98 | TF: [34 en%] | TF: [34 en%] | ||||||||||
| LDL-C: 3.44 | SFAs: 8.5 | SFAs: 16.0 | ||||||||||
| HDL-C: 1.06 | MUFAs: 21.0 | MUFAs: 14.0 | ||||||||||
| TGs: 1.01 | PUFAs: 3.0 | PUFAs: 4.0 | ||||||||||
| 2. (Coconut oil: 22.6 en%) | ↓15.8a M | ↓18.8a M | ↓7.5a M | ↓11.9a M | NR | NR | NR | |||||
| TF: [34 en%] | ↓16.5a F | ↓19.6a F | ↓9.8a F | ↓15.5a F | NR | NR | NR | |||||
| SFAs: 26.0 | ||||||||||||
| MUFAs: 3.7 | ||||||||||||
| PUFAs: 1.0 | ||||||||||||
| Kris-Etherton et al., 1993 (30) | RC (mixed-food diet) | US men, mean age 26 y, NC, n = 18; | 26 | (EV olive oil: 30 en%) | 1. (Butter: 30 en%) | ↓13.6e | ↓18.6e | ↑6.6 | ↓4.5 | NR | ↓7.9 | ↑1.1 |
| TC: NR | TF: [37 en%] | TF: [37 en%] | ||||||||||
| LDL-C: NR | SFAs: 6.0 | SFAs: 21.0 | ||||||||||
| HDL-C: NR | MUFAs: 27.0 | MUFAs: 10.0 | ||||||||||
| TGs: NR | PUFAs: 2.3 | PUFAs: 1.7 | ||||||||||
| 2. (Cocoa butter: 30 en%) | ↓8.5e | ↓11.9e | ↑8.3 | ↓2.3 | NR | ↓5.8 | ↑1.0 | |||||
| TF: [37 en%] | ||||||||||||
| SFAs: 21.0 | ||||||||||||
| MUFAs: 13.0 | ||||||||||||
| PUFAs: 2.1 | ||||||||||||
| Judd et al., 1994 (5) | RC (mixed-food diet) | US men and women, mean age 42.6 y, NC, n = 58; | 32 | (Oleic oil amount NR, may include HO sunflower oil + other oleic food sources) | (Saturated fat amount NR; may include meat, dairy, animal fats, coconut oil) | ↓6.2a | ↓8.2a | ↓3.4a | ↓3.7 | NR | ↓4.1a | ↓1.4a |
| TC: 5.30 | TF: [39 en%] | TF: [39 en%] | ||||||||||
| LDL-C: 3.31 | SFAs: 13.4 | SFAs: 19.3 | ||||||||||
| HDL-C: 1.37 | MUFAs: 16.7 | MUFAs: 11.0 | ||||||||||
| TGs: 1.31 | PUFAs: 6.0 | PUFAs: 6.0 | ||||||||||
| Nestel et al., 1994 (31) | RC (mixed-food diet) | Australian men, mean age 49 y, HC, n = 34; | 21 | (HO sunflower oil: 25 en%) | (Palm oil: 25 en%) | ↓3.5f | ↓4.0f | ↓1.8 | ↓2.3 | NR | NR | NR |
| TC: 5.83 | TF: [36.5 en%] | TF: [37.6 en%] | ||||||||||
| LDL-C: 4.22 | SFAs: 7.7 | SFAs: 11.4 | ||||||||||
| HDL-C: 1.08 | MUFAs: 21.0 | MUFAs: 18.3 | ||||||||||
| TGs: 1.18 | PUFAs: 3.4 | PUFAs: 3.3 | ||||||||||
| Zock et al., 1994 (32) | RC (mixed-food diet) | Dutch men and women, 28–29 y, NC, n = 59; | 21 | (HO sunflower oil: 17.2 en%) | (Fractionated palm oil: 21 en%) | ↓8.8f | ↓12.7f | ↓1.3 | ↓5.0 | NR | ↓7.7f | ↓1.0 |
| TC: 5.06 | TF: [38.5 en%] | TF: [39.6 en%] | ||||||||||
| LDL-C: NR | SFAs: 10.8 | SFAs: 21.0 | ||||||||||
| HDL-C: NR | MUFAs: 21.6 | MUFAs: 12.0 | ||||||||||
| TGs: NR | PUFAs: 4.4 | PUFAs: 4.7 | ||||||||||
| Mata et al., 1996 (33) | NRan, SC (mixed-food diet) | Spanish men (n = 18) and women (n = 24), mean age 45.5 y, NC; | 35 | (Olive oil: 19.3 en%) | (Palm oil + butter: 19.5 en%) | ↓9.8f | ↓11.6a | 0.0 | ↑0.85 | NR | NR | NR |
| TC: NR | TF: [35.2 en%] | TF: [ 35.5] en% | ||||||||||
| LDL-C: NR | SFAs: 9.2 | SFAs: 17.3 | ||||||||||
| HDL-C: NR | MUFAs: 20.9 | MUFAs: 13.6 | ||||||||||
| TGs: NR | PUFAs: 4.0 | PUFAs: 3.6 | ||||||||||
| Cater et al., 1997 (34) | RC (mixed-food diet) | US men, 55–75 y, HC, n = 9; | 21 | (HO sunflower oil: 43 en%) | (Palm oil: 43 en%) | ↓9.8a | ↓14.9a | ↑2.2 | ↑7.4 | NR | NR | NR |
| TC: 5.69 | TF: [53 en%] | TF: [53 en%] | ||||||||||
| LDL-C: NR | SFAs: 6.0 | SFAs: 26.0 | ||||||||||
| HDL-C: NR | MUFAs: 41.0 | MUFAs: 19.0 | ||||||||||
| TGs: 1.52 | PUFAs: 5.0 | PUFAs: 6.0 | ||||||||||
| Cuesta et al., 1998 (35) | NRan, SC (mixed-food diet) | Spanish women, mean age 63 y, HC, n = 14; | 28 | (HO sunflower oil: 29 en%) | (Palm olein: 28.4 en%) | ↓15.0d | ↓17.8d | ↓12.9a | ↑1.2 | 0.0 | ↓15.2h | ↑1.2 |
| TC: 6.41 | TF: [46 en%] | TF: [46 en%] | ||||||||||
| LDL-C: 3.78 | SFAs: 10.0 | SFAs: 19.6 | ||||||||||
| HDL-C: 1.88 | MUFAs: 27.8 | MUFAs: 17.5 | ||||||||||
| TGs: 0.83 | PUFAs: 4.6 | PUFAs: 4.2 | ||||||||||
| Cater and Denke, 2001 (36) | RC (mixed-food diet) | US men, 55–75 y, HC, n = 7; | 21 | (HO sunflower oil: 43 en%) | (Palm oil: 43 en%) | ↓12.3e | ↓16.2e | ↑3.4 | ↓2.8 | NR | NR | NR |
| TC: 5.69 | TF: [53 en%] | TF: [53 en%] | ||||||||||
| LD-CL: 4.00 | SFAs: 6.0 | SFAs: 26.0 | ||||||||||
| HDL-C: 0.93 | MUFAs: 41.0 | MUFAs: 19.0 | ||||||||||
| TGs: 1.52 | PUFAs: 5.0 | PUFAs: 6.0 | ||||||||||
| Judd et al., 2002 (37) | RC (mixed-food diet) | US men, mean age 42 y, NC, n = 50; | 35 | (HO sunflower oil + canola blend: 15 en%) | (Palm stearine + coconut oil + cocoa butter + butter: 26.6 en%) | ↓7.4e | ↓8.5e | ↓4.6e | ↓9.7 | ↓2.3 | ↓4.2 | ↓2.3 |
| TC: 4.77 | TF: [38.2 en%] | TF: [39.7 en%] | ||||||||||
| LDL-C: 3.06 | SFAs: 12.6 | SFAs: 20.7 | ||||||||||
| HDL-C: 1.14 | MUFAs: 17.6 | MUFAs: 10.5 | ||||||||||
| TGs: 1.15 | PUFAs: 6.0 | PUFAs: 6.0 | ||||||||||
| Allman-Farinelli et al., 2005 (38) | RC (mixed-food diet) | Australian men and women, 46–47 y, HC, n = 15; | 35 | (HO sunflower oil, oil amount NR) | (Butter + hard cooking fat, amounts NR) | ↓11.8d | ↓13.5d | ↓5.3a | ↓13.8g | NR | ↓11.4h | ↓0.76 |
| TC: 5.55 | TF: [32.6 en%] | TF: [33.1 en%] | ||||||||||
| LDL-C: NR | SFAs: 8.8 | SFAs: 20.8 | ||||||||||
| HDL-C: NR | MUFAs: 20.3 | MUFAs: 9.6 | ||||||||||
| TGs: 1.45 | PUFAs: 3.5 | PUFAs: 2.7 | ||||||||||
| Tholstrup et al., 2011 (39) | RC (mixed-food diet) | Danish men, 19–64 y, NC, n = 32; | 21 | (Olive oil: 17 en%) | 1. (Lard: 17 en%) | ↓5.8c | ↓10.0c | ↓2.5 | ↑3.4 | ↓2.1 | NR | NR |
| TC: NR | TF: [35 en%] | TF: [35.8 en%] | ||||||||||
| LDL-C: NR | SFAs: 8.0 | SFAs: 14.0 | ||||||||||
| HDL-C: NR | MUFAs: 18.0 | MUFAs: 14.0 | ||||||||||
| TGs: NR | PUFAs: 5.3 | PUFAs: 5.1 | ||||||||||
| 2. (Palm olein: 17 en%) | ↓5.8c | ↓10.4c | ↓1.6 | ↑11.3i | ↓2.4 | NR | NR | |||||
| TF: [35.8 en%] | ||||||||||||
| SFAs: 13.2 | ||||||||||||
| MUFAs: 14.0 | ||||||||||||
| PUFAs: 5.9 | ||||||||||||
| Voon et al., 2011 (40) | RC (mixed-food diet) | Malaysian men and women, 18–30 y, NC, n = 45; | 32 | (EV olive oil: 20.6 en%) | 1. (Palm olein: 20.4 en%) | ↓3.3 | ↓4.3 | ↓2.2 | ↓1.2 | ↓6.0 | ↓4.3 | ↓1.8 |
| TC: 4.71 | TF: [31 en%] | TF: [31 en%] | ||||||||||
| LDL-C: 3.06 | SFAs: 6.6 | SFAs: 12.5 | ||||||||||
| HDL-C: 1.23 | MUFAs: 20.0 | MUFAs: 13.0 | ||||||||||
| TGs: 0.96 | PUFAs: 4.4 | PUFAs: 5.0 | ||||||||||
| 2. (Coconut oil: 20.4 en%) | ↓6.0a | ↓7.3a | ↓6.3a | ↓6.6 | ↓0.5 | ↓4.3 | ↓3.2 | |||||
| TF: [31 en%] | ||||||||||||
| SFAs: 20.6 | ||||||||||||
| MUFAs: 6.9 | ||||||||||||
| PUFAs: 2.9 | ||||||||||||
| Gillingham et al., 2011 (41) | RC (mixed-food diet) | Canadian men and women, mean age 47.5 y, HC, n = 36; | 28 | (HO-canola oil: 25.8 en%) | (Western diet fat blend: 25.8 en%) | ↓6.7a | ↓12.2a | ↓2.9 | ↑11.4 | ↓3.0 | NR | NR |
| TC: 5.94 | TF: [36.8 en%] | TF: [36.8 en%] | ||||||||||
| LDL-C: 3.70 | SFAs: 5.6 | SFAs: 11.2 | ||||||||||
| HDL-C: 1.41 | MUFAs: 23.0 | MUFAs: 16.1 | ||||||||||
| TGs:= 1.84 | PUFAs: 5.7 | PUFAs: 6.5 | ||||||||||
| Substituting HO oils for trans fats | ||||||||||||
| Mensink and Katan, 1990 (25) | RC (mixed-food diet) | Dutch men and women, mean age 26 y, NC, n = 59; | 23 | (HO sunflower + olive oil + rapeseed oils: 9.9 en%) | (High TFA margarine + shortening: 26.1 en%) | ↓5.5c | ↓12.2c | ↑13.6c | ↓13.8c | ↓16.4c | ↓12.2c | ↑7.7c |
| TC: 4.75 | TF: [39.6 en%] | TF: [40.2 en%] | ||||||||||
| LDL-C: 2.18 | SFAs: 9.5 | SFAs: 10.0 | ||||||||||
| HDL-C: 2.38 | MUFAs: 24.0 | MUFAs: 12.6 | ||||||||||
| TGs: 0.96 | PUFAs: 5.0 | PUFAs: 4.6 | ||||||||||
| TFAs: 0.0 | TFAs: 11.0 | |||||||||||
| Judd et al., 1994 (5) | RC (mixed-food diet) | US men and women, mean age 42.6 y, NC, n = 58; | 32 | (Oleic oil amount NR, may include HO sunflower oil + other oleic food sources) | 1. (Trans fat from margarine and shortenings, amounts NR) | ↓4.7a | ↓7.2a | ↑2.9a | ↓11.2a | NR | ↓4.1a | ↑1.4a |
| TC: 5.30 | TF: [39 en%] | TF: [39.1 en%] | ||||||||||
| LDL-C: 3.31 | SFAs: 13.4 | SFAs: 12.7 | ||||||||||
| HDL-C: 1.37 | MUFAs: 16.7 | MUFAs: 11.1 | ||||||||||
| TGs: 1.31 | PUFAs: 6.0 | PUFAs: 6.2 | ||||||||||
| TFAs: 0.7 | TFAs: 6.6 | |||||||||||
| 2. (Trans fat from margarine and shortenings, amounts NR.) | ↓3.7a | ↓5.6e | ↑1.4 | ↓7.2a | NR | ↓2.8a | 0.0 | |||||
| TF: [39.4 en%] | ||||||||||||
| SFAs: 13.0 | ||||||||||||
| MUFAs: 14.1 | ||||||||||||
| PUFAs: 6.0 | ||||||||||||
| TFAs: 3.8 | ||||||||||||
| Judd et al., 2002 (37) | RC (mixed-food diet) | US men, mean age 42 y, NC, n = 50; | 35 | (HO sunflower oil + canola oil blend: 15 en%) | 1. (Trans fat from margarine + shortening: 20.5 en%) | ↓8.0e | ↓12.2e | ↑6.9e | ↓13.9e | ↓13.3e | ↓9.3e | ↑5.0e |
| TC = 4.77 | TF: [38.2 en%] | TF: [39.4 en%] | ||||||||||
| LDL-C: 3.06 | SFAs: 12.6 | SFAs: 12.9 | ||||||||||
| HDL-C: 1.14 | MUFAs: 17.6 | MUFAs: 10.6 | ||||||||||
| TGs: 1.15 | PUFAs: 6.0 | PUFAs: 4.0 | ||||||||||
| TFAs: 0.1 | TFAs: 8.3 | |||||||||||
| 2. (Trans fat from margarine + shortening: 10.8 en%) | ↓7.8e | ↓11.2e | ↑5.7e | ↓16.9e | ↓11.3e | ↓9.3e | ↑5.8e | |||||
| TF: [39.6 en%] | ||||||||||||
| SFAs: 16.9 | ||||||||||||
| MUFAs: 10.6 | ||||||||||||
| PUFAs: 4.3 | ||||||||||||
| TFAs: 4.2 | ||||||||||||
| Lichtenstein et al., 2006 (18) | RC (mixed-food diet) | US men and women, mean age 64 y, HC, n = 30; | 35 | (HO soybean oil: 19.3 en%) | (Partially hydrogenated soybean oil: 19.2 en%) | ↓5.0a | ↓5.6a | ↑3.0 | ↓6.9 | ↓7.4a | ↓5.8a | ↑2.5a |
| TF: [29 en%] | TF: [28.9 en%] | |||||||||||
| TC: 5.75 | SFAs: 5.8 | SFAs: 7.3 | ||||||||||
| LDL-C: 3.86 | MUFAs: 19.0 | MUFAs: 8.0 | ||||||||||
| HDL-C: 1.28 | PUFAs: 2.8 | PUFAs: 8.7 | ||||||||||
| TGs: 1.63 | TFAs: 0.33 | TFAs: 2.5 |
Changes labeled with a letter are significant: aP < 0.05; bP < 0.005; cP < 0.0001; dP < 0.001; eP < 0.01; fP < 0.02; gP < 0.007; hP < 0.027; iP < 0.03. en%, percentage of energy; EV, extra virgin; F, female; HC, hypercholesterolemic (LDL: ≥3.36 mmol/L) as reported or calculated according to Freidwald et al. (42); HDL-C, HDL cholesterol; HO, high oleic acid; LDL-C, LDL cholesterol; M, male; NC, normocholesterolemic; NR, not reported; Nran, nonrandomized; RC, randomized crossover; SC, sequential crossover; TC, total cholesterol; TC:HDL-C, ratio of total cholesterol to HDL cholesterol; TF, total fat; TFA, trans FA; %∆, percentage of change.
Substituting HO oils for fats high in saturated fat
Clinical evidence.
A total of 17 controlled crossover intervention studies were analyzed (5, 25, 26, 28–41), consisting of 23 diet comparisons (DCs) and involving a total of 528 men and women, compared the blood lipid effects of substituting defined amounts of HO oils (i.e., >70% oleic acid) for defined amounts of fats and oils high in saturated fat (Table 2). The mean ± SD subject age was 29 ± 16 y, diet duration was 29 ± 7 d; 8 studies used hypercholesterolemic and 9 used normocholesterolemic subjects. In all studies, dietary SFA amounts were substituted with isocaloric or near isocaloric amounts of MUFAs, and in most (26, 28–36, 39–41) but not all (5, 25, 37, 38), the SFA test fats were substituted with equivalent amounts of HO oils. All studies used mixed-food diets that provided total fat amounts ranging from 30% to 53% of energy (mean: 38.6% of energy), of which the contribution of the saturated and HO oil test fats ranged from 25% to 85% of the total dietary fat (mean: 64.6%). Some studies described the baseline diets as habitual diets or usual diets with defined amounts of total fat that ranged from 30% to 46% of energy and SFAs from 10% to 21% of energy (30, 35, 33) or as a defined standardization diet (40). Most studies, however, did not adequately describe the subjects’ baseline diets before the experimental diet periods. Studies that compared the blood lipid effects of HO oils solely with baseline or habitual diets were not included.
The result of substituting HO oils (i.e., >70% oleic acid) for fats and oils high in SFAs on plasma lipids and lipoproteins are summarized in Table 3. Predictably, of the 23 DCs (5, 25, 26, 28–41), 20 showed that replacing food fats high in SFAs (e.g., lard, butter, palm oil) with isocaloric amounts of MUFAs from HO oils such as olive oil, HO sunflower oil, and HO safflower oil resulted in significant reductions in plasma TC (WMC: −8.8%; P < 0.05) and LDL cholesterol (WMC: −12.1%; P < 0.05) in normocholesterolemic and moderately hypercholesterolemic men and women. When results from all DCs are considered (n = 23), the WMC in TC and LDL was −8.0% and −10.9%, respectively (Table 3). No significant changes were reported in HDL cholesterol in 15 of 23 DCs (WCM: −0.36%), whereas significant reductions were reported in 7 DCs (WMC: −2.8%; P < 0.05) (5, 29, 37, 38, 40, 35), and 1 DC showed a significant increase of 5.6% (P < 0.001) (28). The WMC in HDL for all DCs combined (n = 23) was −1.9%. No significant changes in TGs was observed in 17 of 23 DCs (WMC: −2.3%), whereas 4 DCs showed significant reductions in TGs, ranging from −11.9% to −15.5% (WMC: −13.7%; P < 0.05) (25, 29, 38), and 2 DCs reported increases (9.5% and 11.3%, respectively; P < 0.05) (28, 39). Overall, the WMC in TGs from all 23 DCs was −3.0% (Table 3). Consistent with the observed reductions in LDL, substituting HO oils for fats and oils high in SFAs reduced apoB by a WMC of −7.9% in all 12 DCs that examined this marker with 7 DCs reporting significant reductions (WMC: −9.8%; P < 0.05) (5, 25, 26, 28, 32, 35, 38) and 5 DCs reporting no significant changes (WMC: −4.8%) (30, 37, 40). In contrast, 10 of the same 12 DCs exhibited no change in apoA-1 with all 12 DCs, resulting in a WMC of −0.90%. In those studies that assessed changes in TC:HDL cholesterol, 7 of 8 DCs showed no significant changes (WMC: −2.5%) (34, 37, 39–41), whereas 1 study showed a substantial reduction of 10% (P < 0.0001) (25). Taken together, all DCs that measured TC:HDL cholesterol resulted in a combined WMC of −4.0% (Table 3). In a further analysis, when results from the studies that reported TC:HDL cholesterol (25, 35, 37, 39–41) are combined with calculated changes from studies that did not (5, 26, 28–34, 36, 38), 19 of 23 DCs showed a reduction in TC:HDL cholesterol with a mean reduction of −6.2% (range: 1.8% to −17.9%), suggesting a more consistent and favorable reduction in this ratio than is revealed from the DCs in Table 3. These observations are consistent with a meta-analysis of 35 RCTs in which the investigators predicted that each 1% energy replacement of SFAs with MUFAs would decrease TC:HDL cholesterol by 0.029 (21). The observed changes in Table 3 for TC, LDL cholesterol, HDL cholesterol, and TGs are also, in general, consistent with the predicted changes from this meta-analysis (21).
TABLE 3.
Summary of results from controlled clinical trials that assessed the effects of substituting HO oils for fats and oils high in SFAs, trans fats, or n–6 PUFAs on plasma lipids and lipoproteins1
| HO oils for saturated fats | HO oils for trans fats | HO oils for PUFA oils | |
| TC | Sig.↓ in 20 of 23 DCs | Sig.↓ in 6 of 6 DCs | NS∆ in 6 of 11 DCs |
| mean %∆ | −8.0 | −5.7 | 2.7 |
| LDL-C | Sig.↓ in 20 of 23 DCs | Sig. ↓ in 6 of 6 DCs | NS∆ in 7 of 11 DCs |
| mean %∆ | −10.9 | −9.2 | 1.4 |
| HDL-C | NS∆ in 15 of 23 DCs | Sig.↑ in 4 of 6 DCs | NS∆ in 7 of 11 DCs |
| mean %∆ | −1.9 | 5.8 | 6.6 |
| TGs, % | NS∆ in 17 of 23 DCs | Sig.↓ in 5 of 6 DCs | NS∆ in 9 of 11 DCs |
| mean %∆ | −3.0 | −11.9 | 5.6 |
| TC:HDL-C | NS∆ in 7 of 8 DCs | Sig.↓ in 4 of 4 DCs | NS∆ in 4 of 5 DCs |
| mean %∆ | −4.0 | −12.1 | −3.8 |
| apoB | Sig.↓ in 7 of 12 DCs | Sig.↓ in 6 of 6 DCs | NS∆ in 4 of 9 DCs2 |
| mean %∆ | −7.9 | −7.3 | 2.8 |
| apoA-1 | NS∆ in 10 of 12 DCs | Sig. ↑ in 5 of 6 DCs | NS∆ in 7 of 9 DCs |
| mean %∆ | −0.9 | 3.7 | 3.1 |
DC, dietary comparison; HDL-C, HDL cholesterol; HO, high oleic oil; LDL-C, LDL cholesterol; mean %∆, weighted mean change from the total number of DCs indicated in each above adjoining row; NS∆, nonsignificant change; PUFA, n–6 polyunsaturated FA (primarily linoleic acid); Sig.↓, significant reduction at least P < 0.05; Sig.↑, significant increase at least P < 0.05; TC, total cholesterol.
ApoB results were inconsistent as 4 studies showed a significant increase, 4 no change, and 1 a significant decrease.
It is noteworthy that 2 RCTs in Malaysian men and women failed to demonstrate significant reductions in TC and LDL cholesterol after replacing 6.0% and 7.5% of energy of SFAs from palm olein-rich high-SFA diets (12.5% and 16.0% of energy from SFAs, respectively) with equivalent amounts of MUFAs from olive oil (29, 40). Conversely, 2 other RCTs, similar in design and amounts of SFAs from palm olein-rich diets replaced by MUFAs from HO sunflower oil and olive oil, in Spanish women (35) and Dutch men (39), respectively, showed significant reductions in TC (P < 0.001) and LDL cholesterol (P < 0.001). Ethnic differences notwithstanding, reasons for these inconsistent findings involving the replacement of SFAs from palm olein with MUFAs from HO oils are unclear.
Other studies that used different dietary approaches assessed the blood lipid effects of replacing SFAs with equivalent amounts of MUFAs from HO oils. For example, 3 randomized crossover studies compared the effects of replacing SFAs from palm oil with MUFAs from HO safflower oil or HO sunflower oil in which patients consumed liquid formula diets that contained 40% of energy total fat provided by a single test fat (23, 24, 27). Consistent with the findings from the mixed-food diet studies, these studies all demonstrated significant reductions in TC (P < 0.05) and LDL (P < 0.05) (mean: −9.7% and −15.7%, respectively), with no significant changes in HDL cholesterol or TGs (−2.2% and −3.2%, respectively) (Table 2). In another crossover controlled feeding trial (5), the blood lipid effects of a diet high in oleic acid (16.7% of energy), provided mainly from HO sunflower oil, to an isocaloric amount of C12:0 + C14:0 + C16:0 SFAs were assessed. Although the percentage of total dietary fat provided by the HO sunflower oil and SFA test fats used in the diets was not described, observed reductions in TC, LDL cholesterol, HDL cholesterol, TGs, apoB, and apoA1 were comparable with other studies reviewed herein, suggesting that the amount of HO sunflower oil and SFA test fats used also were comparable. Taken as a whole, these results are consistent with a review (43) that reported outcomes from 12 studies (44–55) which showed that substituting diets that contained high SFA food sources such as milk fat, palm oil, or coconut oil with MUFAs from canola oil significantly reduced TC and LDL cholesterol by a mean of −11.4% and −15.8%, respectively, whereas HDL cholesterol and TGs were not significantly altered in all but 1 study.
In summary, in clinical studies that assessed the replacement of SFA food sources with equivalent amounts of HO oils (i.e., >70% oleic acid), most showed significant reductions in TC, LDL cholesterol, and apoB but without significant changes in HDL cholesterol, TGs, TC:HDL cholesterol, or apoA-1.
Observational evidence.
Although no RCTs have tested the effects of replacing SFAs with HO oil-containing MUFA diets on CHD outcomes, prospective cohort studies have assessed the association of consuming MUFAs compared with SFAs on CHD risk. In a 14-y prospective study of 80,082 female nurses that estimated changes in the risk of CHD associated with the isocaloric substitution of specific classes of FAs (56), replacing 5% of energy from SFAs with an isocaloric amount from unsaturated fats (i.e., MUFAs + PUFAs) was associated with 42% lower risk (95% CI: 23%, 56%; P < 0.001), and replacing 5% of energy from SFAs with an equivalent amount of MUFAs alone reduced risk by a similar degree. In a follow-up study among 5672 women from the Nurses’ Health Study with type 2 diabetes (57), replacing 5% of energy from SFAs with an equivalent amount of MUFAs was associated with a 37% lower risk of CVD (95% CI: 0%, 60%; P = 0.048). In a review that estimated the effects of dietary fat consumption on CHD risk on the basis of TC:HDL cholesterol changes from short-term clinical trials (58), coupled with observed associations between TC:HDL cholesterol and CHD outcomes from cohort studies (59), researchers estimated that replacing SFAs with MUFAs was associated with a reduced relative risk of CHD (RR: 0.93; 95% CI: 0.89, 0.96) (60). In contrast, a pooled analysis from 11 American and European cohort studies that consisted of 344,696 individuals (71% women) and 4–10 y of follow-up observed an increased risk of CHD when 5% of energy from MUFAs was substituted for an equivalent amount of SFAs (HR: 1.19; 95% CI: 1.00, 1.42) (61). However, as noted by these researchers, the results may be due in part to the intake of TFAs which was included in the sum of MUFAs despite an adjustment for TFAs in most, but not all, of the cohort studies. Moreover, the main food source of MUFAs in these studies is animal fat, and MUFA intake is highly correlated with SFA intake and moderately correlated with TFA intake, such that residual confounding because of meat, dairy, and hydrogenated vegetable oils cannot be excluded (62).
Substituting HO oils for trans fat
Clinical evidence.
Four randomized controlled crossover intervention trials that consisted of 6 DCs were identified that compared blood lipid effects of substituting HO oils compositionally similar with H-OSBO (i.e., >70% oleic acid) for specified amounts of TFA-containing PHVOs (5, 18, 25, 37) (Table 2). The studies included a total of 197 men and women with a mean age of 43 ± 15 y, diet duration of 31 ± 5 d; 3 studies used normocholesterolemic subjects (5, 25, 37) and 1 used hypercholesterolemic subjects (18). In 4 DCs, dietary TFAs (range: 3.1–11% of energy) was substituted by an equivalent or near equivalent amount of MUFAs (5, 25, 37) from HO oils, whereas 1 DC compared higher amounts of MUFAs with somewhat lower amounts of TFAs (i.e., 7% of energy compared with 4.1% of energy, respectively) (37). One DC, by design, compared the blood lipid effects of H-OSBO with an equivalent amount of PHSBO whereby each accounted for 66.6% of total dietary fat, resulting in unequal amounts of MUFAs compared with TFAs (i.e., 11% of energy from MUFAs compared with 2.2% of energy from TFAs, respectively) (18). All of the DCs used mixed-food diets that provided total fat amounts that ranged from 29% to 40% of energy, of which the contribution of PHVO and HO oil test fats ranged from 27% to 66.6% and from 25% to 66.6% of total dietary fat, respectively. In all studies, diets were administered under controlled feeding conditions in which subjects consumed ≥1 meal on site with all other food provided and consumed off site.
Table 2 shows the results of studies that assessed the blood lipid effects of substituting HO oils that contained >70% oleic acid for TFA-containing PHVOs. Not surprisingly, all 6 DCs showed that replacing TFA-containing PHVOs with HO oils (i.e., olive oil, HO sunflower oil, and H-OSBO) significantly lowered plasma TC (P < 0.05) and LDL cholesterol (P < 0.05) (WMC: −5.7% and −9.2%, respectively) in normocholesterolemic and moderately hypercholesterolemic men and women (Table 3). In addition, all 6 DCs showed directional increases in HDL cholesterol (WMC: 5.8%) with 4 of 6 DCs exhibiting significant increases (P < 0.05) (WMC: 7.3%). All 6 DCs also consistently demonstrated a decrease in TGs (WMC: −11.9%) with 5 of 6 DCs resulting in significant reductions (P < 0.05) (WMC: −12.6%). In agreement with the LDL- and HDL cholesterol changes, all DCs showed significant reductions in apoB (P < 0.05) (WMC: −7.3%). Most (5 of 6 DCs) showed significant increases in apoA-1 (P < 0.05) (WMC: 4.5%); whereas all 6 DCs combined resulted in a WMC of 3.7%. Concurrent with the observed decreases in TC and increases in HDL cholesterol, 4 of 4 DCs that measured changes in TC:HDL cholesterol reported significant reductions (18, 25, 37), ranging from −7.4% to −16.4% (P < 0.05) (WMC: −12.1%). Taken as a whole these results are consistent with a meta-analysis of 13 RCTs which showed that for each 1% energy replacement of TFAs with MUFAs led to significant reductions in TC, LDL cholesterol, apoB, TGs, and TC:HDL cholesterol, along with increases in HDL cholesterol and apoA-1 (21). Furthermore, these researchers estimated, based on the effects of TFA, SFA, MUFA, and PUFA consumption on plasma biomarkers of CVD risk from controlled clinical trials, that replacing 7.5% of energy from PHVOs containing 20% TFAs with HO sunflower oil would decrease CHD risk by 9.2%.
In summary, in a limited number of RCTs that assessed replacing TFA-containing PHVOs with HO oils (>70% oleic acid), all or most showed significant reductions in TC, LDL cholesterol, apoB, TGs, and TC:HDL cholesterol and significant increases in HDL cholesterol, and apoA-1.
Observational evidence.
In a prospective study of female nurses from the Nurses’ Health Study (56), researchers estimated that, compared with equivalent energy from carbohydrates, the relative risk of CHD for a 2% of energy dietary replacement from TFAs was 1.93 (95% CI: 1.43, 2.61; P < 0.001), whereas replacing 2% of energy from TFAs with the same amount of unsaturated fats (i.e., MUFAs + PUFAs) was associated with a 53% lower risk, and, when replaced only by MUFAs, an apparent similar degree of risk reduction was observed (56). In a pooled analysis that included results from the Nurses’ Health Study and another large prospective cohort study (21), researchers estimated that replacing 7.5% of energy from PHVOs with either HO sunflower oil or SBO reduced CHD risk by 15.9% and 21.8% respectively.
Substituting HO oils for high-PUFA oils
Clinical evidence.
Twelve controlled clinical intervention studies compared the blood lipid effects of substituting HO oils compositionally similar to H-OSBO (i.e., >70% oleic acid) for equivalent amounts of oils high in PUFAs (primarily LA) (Table 4). The studies included a total of 369 men and women aged 18–69 y with dietary treatments that ranged from 21 to 84 d (mean: 36 d); 7 studies used normocholesterolemic (28, 30, 33, 63–65, 67) and 5 used hypercholesterolemic (18, 23, 66, 68, 69) subjects. In all of the studies, the high-PUFA test fats were substituted with equivalent or near equivalent amounts of HO oils, and in most (23, 28, 30, 63–66, 68, 69), but not all studies (18), dietary PUFAs were substituted with equivalent amounts of MUFAs. In the latter study the blood lipid effects of H-OSBO was compared with an isocaloric amount of SBO, resulting in a somewhat higher substitution of MUFAs compared with PUFAs (12.4% of energy from MUFAs compared with 9.9% of energy from PUFAs, respectively) (18). Most studies used mixed-food diets that provided total fat amounts that range from 29% to 40% of energy (mean: 34.9% of energy), of which the contribution of the PUFA oils to total dietary fat ranged from 20.4% to 81% (mean: 54.9%) and HO oils ranged from 19.5% to 81% (mean: 56.3%). In one crossover study, researchers used liquid formula diets (23), wherein the test fats were the sole source of fat calories in 40% of energy fat diets.
TABLE 4.
Randomized controlled dietary trials assessing the effect of the direct substitution of high oleic oils for high linoleic oils on plasma lipids and lipoproteins1
| Author, year, (reference) | Design (type of diet) | Subjects/ baseline blood lipids, mmol/L | Duration of diet, d | HO diet (en%: from test fat) [diet en%] | Control oil diet (en%: from test fat) [diet en%] | TC, %Δ | LDL-C, %Δ | HDL-C, %Δ | TGs, %Δ | TC:HDL-C, %Δ | apoB, %Δ | apoA-1, %Δ |
| Mattson and Grundy, 1985 (23) | RC (liquid-diet) | US adults, 47–69 y, HC, n = 20; | 28 | (HO safflower oil: 40 en%) | (Safflower oil: 40 en%) | ↑3.1 | ↓0.8 | ↓8.5 | ↑7.8 | NR | NR | NR |
| TC: 6.80 | TF: [40 en%] | TF: [40 en%] | ||||||||||
| LDL-C: NR | SFAs: 3.4 | SFAs: 4.5 | ||||||||||
| HDL-C: NR | MUFAs: 29 | MUFAs: 5.9 | ||||||||||
| TGs: NR | PUFAs: 7 | PUFAs: 29.3 | ||||||||||
| Mensink and Katan, 1989 (63) | Par (mixed-food diet) | Dutch men and women, mean age 26 y, NC, n = 58; | 36 | (Olive oil: 7.3 en%) | (Sunflower oil: 7.7 en%) | ↓4.4a | ↓5.0a | ↓3.5 | ↑4.1 | NR | ↓6.2a | ↑0.6 |
| TC: 4.81 | TF: [37.4 en%] | TF: [37.6en%] | ||||||||||
| LDL-C: 3.27 | SFAs: 12.9 | SFAs: 12.6 | ||||||||||
| HDL-C: 1.34 | MUFAs: 15.1 | MUFAs: 10.8 | ||||||||||
| TGs: 0.99 | PUFAs: 7.9 | PUFAs: 12.7 | ||||||||||
| Mata et al., 1992 (64)2 | NRan, SC (mixed-food diet) | Spanish men, n = 46, mean age 33 y (18–61 y), NC; | 84 | (Olive oil: 20.7 en%) | (Sunflower oil: 18.0 en%) | ↓1.0 | ↓11.3 | ↑17.6b | ↑2.0 | ↓12.0c | NR | NR |
| TC: NR | TF: [37 en%] | TF: [37 en%] | ||||||||||
| LD-C: NR | SFAs: 13.2 | SFAs: 12.7 | ||||||||||
| HDL-C: NR | MUFAs: 19.7 | MUFAs: 11.2 | ||||||||||
| TGs: NR | PUFAs: 3.5 | PUFAs: 12.4 | ||||||||||
| Mata et al., 1992 (28) | NRan, SC (mixed-food diet) | Spanish women, mean age 43 y, NC, n = 21; | 42 | (Olive oil: 21.0 en%) | (Sunflower oil: 21.0 en%) | ↑1.5 | ↓5.1 | ↑11.9b | ↑10.5h | NR | ↑6.5b | ↑12.3b |
| TC: 4.94 | TF: [ 35.4 en%] | TF: [35.3 en%] | ||||||||||
| LDL-C: 2.92 | SFAs: 10.3 | SFAs: 9.9 | ||||||||||
| HDL-C: 1.55 | MUFAs: 21.4 | MUFAs: 12.4 | ||||||||||
| TGs: 0.96 | PUFAs: 3.3 | PUFAs: 12.5 | ||||||||||
| Wahrburg et al., 1992 (65) | RC (mixed-food diet) | German men, n = 21, and n = 17 women, 21–30 y, NC; | 28 | (Olive oil: 21.7 en%) | (Sunflower oil: 21.7 en%) | ↑1.5 | 0.0 | ↑2.2 | ↑5.0 | NR | ↑1.0 | ↑4.5b |
| TC: 5.17 | TF: [32.6 en%] | TF: [32.6 en%] | ||||||||||
| LDL-C: NR | SFAs: 10.2 | SFAs: 10.1 | ||||||||||
| HDL-C: NR | MUFAs: 16.0 | MUFAs: 9.9 | ||||||||||
| TGs: 1.13 | PUFAs: 4.1 | PUFAs: 10.3 | ||||||||||
| Kris-Etherton et al., 1993 (30) | RC (mixed-food diet) | US men, mean age 26 y, NC, n = 18; | 26 | (EV olive oil: 30 en%) | (Soybean oil: 30 en%) | ↑9.4d | ↑10.8 | ↑6.6 | ↑15.1 | NR | ↑17.4d | ↑4.0 |
| TC: NR | TF: [37.0 en%] | TF: [37.0 en%] | ||||||||||
| LDC-C: NR | SFAs: 6.0 | SFAs: 6.3 | ||||||||||
| HDL-C: NR | MUFAs: 27.2 | MUFAs: 10.1 | ||||||||||
| TGs: NR | PUFAs: 2.3 | PUFAs: 17.8 | ||||||||||
| Lichtenstein et al., 1993 (66) | RC (mixed-food diet) | US men and women, mean age 61 y, HC, n = 15; | 32 | (Olive oil: 20 en%) | (Corn oil: 20 en%) | ↑5.7d | ↑5.6 | 0.0 | ↑3.7 | ↑1.5 | ↑5.1 | 0.0 |
| TC: 5.71 | TF: [30.0 en%] | TF: [29.4en%] | ||||||||||
| LDL-C: 3.93 | SFAs: 6.9 | SFAs: 6.9 | ||||||||||
| HDL-C: 1.24 | MUFAs: 17.0 | MUFAs: 9.0 | ||||||||||
| TGs: 1.21 | PUFAs: 3.9 | PUFAs: 11.2 | ||||||||||
| Carmena et al., 1996 (67) | NRan, SC (mixed-food diet) | Spanish men, n = 18 mean age 57.3 y, NC; | 21 | (Olive oil: 30.5 en%) | (Sunflower oil: 31.0 en%) | ↑17.5b | ↑21.4b | ↑10.0a | 0.0 | NR | ↑10.8d | ↑2.5 |
| TC: NR | TF: [30.5 en%] | TF: [31.0en%] | ||||||||||
| LDL-C: NR | SFAs: 6.9 | SFAs: 6.8 | ||||||||||
| HDL-C: NR | MUFAs: 21.6 | MUFAs: 10.9 | ||||||||||
| TGs: NR | PUFAs: 4.7 | PUFAs: 13.3 | ||||||||||
| Mata et al., 1996 (33) | NRan, SC (mixed-food diet) | Spanish men, n = 24 and women, n = 18, mean age 45.5 y, NC; | 35 | (Olive oil: 19.3 en%) | (Sunflower oil: 17.8 en%) | ↑5.0b | ↑4.2b | ↑6.1b | ↑14.4b | NR | NR | NR |
| TC: NR | TF: [35.2 en%] | TF: [35.6en%] | ||||||||||
| LDL-C: NR | SFAs: 9.2 | SFAs: 9.5 | ||||||||||
| HDL-C: NR | MUFAs: 20.9 | MUFAs: 12.0 | ||||||||||
| TGs: NR | PUFAs: 4.0 | PUFAs: 12.5 | ||||||||||
| Pedersen et al., 2000 (68) | RC (mixed-food diet) | Danish men, 20–28 y, HC, n = 18; | 21 | (EV olive oil: 19 en%) | (Sunflower oil: 19 en%) | ↑10.9 | ↑14.2b | ↑7.7 | ↑19.4 | ↑3.0 | ↑15.1d | ↑8.4 |
| TC: 4.74 | TF: [35.0 en%] | TF: [35.0 en%] | ||||||||||
| LDL-C: 3.40 | SFAs: 11.0 | SFAs: 10.0 | ||||||||||
| HDL-C: 1.10 | MUFAs: 21.0 | MUFAs: 9.0 | ||||||||||
| TGs: 1.20 | PUFAs: 3.0 | PUFAs: 15.0 | ||||||||||
| Thijssen and Mensink, 2005 (69) | RC (mixed-food diet) | Dutch men, n = 18 and 27 women, 18–65 y, HC; | 35 | (Olive oil + HO sunflower oil: 22.6 en%) | (Safflower oil: 22.6 en%) | ↑1.2 | ↑1.6 | 0.0 | ↑0.08 | ↑0.07 | ↑2.0 | ↑0.07 |
| TC: 6.04 | TF: [37.7 en%] | TF: [38.0en%] | ||||||||||
| LDL-C: 4.33 | SFAs: 11.0 | SFAs: 11.2 | ||||||||||
| HDL-C: 1.48 | MUFAs: 19.1 | MUFAs: 12.5 | ||||||||||
| TGs: 1.15 | PUFAs: 5.0 | PUFAs: 11.8 | ||||||||||
| Lichtenstein et al., 2006 (18) | RC (mixed-food diet) | US men and women, n = 30, mean age 64 y, HC; | 35 | (HO soybean oil: 19.3 en%) | (Soybean oil: 20.8 en%) | ↑0.17 | ↑1.1 | ↑3.0 | ↓3.6 | ↓3.9 | ↓1.0 | ↑2.5 |
| TC: 5.75 | TF: [29.0 en%] | TF: [31.2 en%] | ||||||||||
| LDL-C: 3.86 | SFAs: 6.5 | SFAs: 6.5 | ||||||||||
| HDL-C: 1.28 | MUFAs: 18.9 | MUFAs: 6.5 | ||||||||||
| TGs: 1.63 | PUFAs: 2.8 | PUFAs: 12.7 |
Changes labeled with a letter are significant: aP < 0.05; bP < 0.001; cP < 0.005; dP < 0.01. apoA-1, apolipoprotein A-1; apoB, apolipoprotein B; en%, percentage of energy; EV, extra virgin; HC, hypercholesterolemic (LDL ≥3.36 mmol/L), as reported or calculated according to Freidwald et al. (42); HDL-C, HDL cholesterol; HO, high oleic acid; LDL-C, LDL cholesterol; NC, normocholesterolemic; NR, not reported; NRan, nonrandomized; Par, parallel; RC, randomized crossover; SC, sequential crossover; TC, total cholesterol; TF, total fat; %∆, percentage of change.
Reported effects in women were excluded due to unequal treatment exposure times (days) for MUFAs and PUFAs.
Summarized in Table 3 are the results of 11 studies that assessed the effect of substituting HO oils for equivalent or near equivalent amounts of high-PUFA oils on plasma lipids and lipoproteins in subjects that consumed the test fat as part of mixed-food diets (18, 28, 30, 33, 63–69). Six of 11 studies exhibited no significant changes in TC (WMC: 2.1%) (18, 28, 64, 65, 68, 69), whereas 4 studies resulted in a significant increase (P < 0.05) (WMC: 8.4%) (30, 33, 66, 67), and 1 showed a significant decrease (−4.4%; P < 0.05) (63). Pooling the results from all studies resulted in a WMC in TC of 2.7%. Similarly, 7 of 11 DCs showed no significant changes in LDL cholesterol (WMC: −0.20%) (18, 30, 28, 64, 65, 66, 69), whereas 3 studies showed a significant increase (P < 0.05) (WMC: 10.5%) (33, 67, 68), and 1 study exhibited a significant decrease (−5.0%; P < 0.05) (63). The combined results from all studies showed a WMC in LDL cholesterol of 1.4% (Table 3). No significant change in HDL cholesterol was observed in 7 of 11 studies (WMC: 1.0%) (18, 30, 63, 65, 66, 68, 69) whereas 4 studies exhibited significant increases in HDL cholesterol (P < 0.05) (WMC: 11.8%) (28, 33, 64, 67). The combined results from all 11 studies showed an increase in HDL cholesterol (WMC: 6.6%). No significant change in plasma TGs was observed in 9 of 11 studies (WMC: 3.9%), whereas 2 studies exhibited significant increases (10.5%, P < 0.05 and 14.4%, P < 0.05, respectively) (28, 33). The combined results from all studies showed an overall WMC of 5.6% in TGs. In the 5 studies that measured TC:HDL cholesterol (18, 52, 55–57), 4 showed no significant changes (WMC: 0.3%), whereas 1 crossover study (64) reported a significant decrease in TC:HDL cholesterol of −12.0% (P < 0.005). Combining outcomes from all 5 studies showed a WMC in TC:HDL cholesterol of −3.8%. In the 9 studies that reported apoB results (18, 28, 30, 65–69), most showed changes that were directionally consistent with the changes observed for LDL cholesterol (30, 63, 66–69). However, the observed amounts of change in apoB were mixed, wherein 4 studies showed relatively small, nonsignificant alterations (WMC: 1.4%) (18, 65, 66, 69), and 4 reported relatively large and significant increases (P < 0.05) (WMC: 12.2%) (30, 28, 67, 68), and 1 showed a significant reduction (−6.2%; P < 0.05) (63). All studies taken together showed a WMC in apoB of 2.8% (Table 3). Similarly, in the 9 studies that reported apoA-1 results (18, 28, 30, 62, 64–68), most showed changes that were directionally consistent with the changes observed for HDL cholesterol (18, 28, 30, 65–69). Seven of 9 studies showed little change in apoA-1 (WMC: 1.9%) (18, 30, 63, 66–69), whereas 2 studies showed a significant increase (12.3%, P < 0.001 and 4.5%, P < 0.001, respectively) (28, 65). All studies combined exhibited a WMC for apoA1 of 3.1%. In general, the findings in this review of substituting HO oils for high-PUFA oils are directionally consistent with a meta-analysis of 35 clinical trials which showed that each 1% of energy isocaloric replacement of MUFAs with PUFAs resulted in significant reductions in TC and LDL cholesterol (P < 0.05), and nonsignificant changes in HDL cholesterol, TGs, TC:HDL cholesterol, apoB, and apoA-1 (21).
In summary, in 11 controlled intervention studies that examined the plasma lipid and lipoprotein effects of substituting HO oils (i.e., >70% oleic acid) for equivalent amounts of oils high in PUFAs (mainly LA), most showed nonsignificant change in TC, LDL cholesterol, HDL cholesterol, TGs, apoB, apoA-1, and TC:HDL cholesterol, albeit the directional changes shown in Table 3 suggest that oils high in n–6 PUFAs may have a slightly more favorable effect.
Observational evidence.
In a meta-analysis that assessed the effect of replacing TFA-containing PHVOs with high-MUFA or high-PUFA vegetable oils on CHD risk, researchers estimated, based on blood lipids and lipoprotein results from 13 controlled clinical trials, that replacing 7.5% of energy from PHVOs with HO sunflower oil or SBO reduced risk by 9.2% and 8.8%, respectively (21). When the effects were estimated on the basis of CHD outcomes from prospective cohort studies, the magnitude of CHD risk reduction was 15.9% and 21.8%, respectively. The researchers noted that it was not clear whether the predicted CHD risk reductions based on clinical trials is an underestimation or if the reductions based on cohort studies is an overestimation. Other cohort studies have assessed the association between specific dietary FAs and CVD risk. In the Nurses’ Health Study (56), when modeling an isocaloric replacement of 2% of energy from TFAs with either MUFAs or PUFAs after adjustments for dietary confounders, including SFAs, PUFAs, MUFAs, TFAs, cholesterol, protein, calories, fiber, and nondietary covariates, both were associated with a similar marked reduction in CHD risk. Likewise, when modeling the isocaloric replacement of 5% of energy from SFAs with MUFAs or PUFAs, each resulted in substantial reductions in CHD risk with PUFAs having a somewhat greater effect. In contrast, a pooled analysis of 11 cohort studies (61), showed a significant reduction in the risk of coronary events (HR: 0.87; 95% CI: 0.77, 0.97) and coronary deaths (HR: 0.74; 95% CI: 0.61, 0.89) when 5% of energy from PUFAs replaced an equivalent amount of SFAs, whereas, when the same amount of MUFAs were substituted for SFAs, there was an indication of a direct association with the risk of coronary events (HR: 1.19; 95% CI: 1.00, 1.42) but not between substitution of MUFAs and risk of coronary deaths (HR: 1.01; 95% CI: 0.73, 1.41). The researchers noted that the indication of an increased risk of CHD associated with a lower intake of SFAs and an isocalorically higher intake from MUFAs may be due in part to the intake of TFAs, which was included in the sum of MUFAs. Furthermore, the main sources of MUFAs was animal fat, whereby confounding from other dietary components in meat and dairy products cannot be excluded. As noted by others (62), results from prospective trials are challenging to interpret because MUFA intake is highly correlated with SFA intake and is moderately correlated with intakes of PUFAs and TFAs (56), suggesting that caution is warranted in the interpretation of such results.
Discussion
H-OSBO is a trait-enhanced oil high in oleic acid (>72–75%) and low in n–6 and n–3 PUFAs and SFAs (70, 71). The primary goal of these trait enhancements was to create an oil with superior functional properties, including high heat and oxidative stability for use in the food service and food manufacturing industry as an alternative to partially hydrogenated vegetable oils (72). Regular SBO is the dominant edible oil in the marketplace and is a primary contributor of PUFAs in the US diet (73). H-OSBO is predicted to replace a substantial portion of US SBO in the near future. In view of the lack of information about the health effects of H-OSBO, this review was undertaken to provide insight into the potential benefits of H-OSBO on CHD risk by assessing the available evidence from dietary RCTs on the effects of substituting HO oils compositionally similar to H-OSBO for equivalent amounts of fats and oils rich in SFAs, TFAs, and PUFAs on qualitative and quantitative changes in plasma lipids and lipoproteins.
In the present review of controlled intervention studies in which diets high in SFA sources (e.g., butter, lard, palm oil, coconut oil were substituted with equivalent amounts of HO oils compositionally similar to H-OSBO (i.e., >70% oleic acid), most studies predictably showed significant reductions in plasma TC, LDL cholesterol, and apoB but with little change in HDL cholesterol, TGs, TC:HDL cholesterol, or apoA-1. Support for these findings was shown in a review of the blood lipid effects of canola oil in which nearly all of the studies showed that substituting diets high in SFA food sources with high MUFAs from canola oil (62% MUFAs, 28% PUFAs, 7% SFAs) significantly reduced TC and LDL cholesterol, whereas HDL cholesterol and TGs were not significantly altered (43). In addition, prospective cohort studies that assessed the effect of consuming MUFAs compared with SFAs on CHD risk in female nurses showed a reduction in risk when 5% of energy was substituted for an equivalent amount of SFAs (56). In a study among nurses with type 2 diabetes, researchers estimated that replacing 5% of energy from SFAs with an equivalent amount of MUFAs was associated with a 37% lower risk of CVD (57). Conversely, results from a pooled analysis of 11 cohort studies showed an indication of an increased risk of coronary events when 5% of energy from MUFAs was substituted for an equivalent amount of SFAs (61). The researchers noted, however, that their results may be influenced in part by the intake of TFAs which was included in the sum of MUFAs despite an adjustment for TFAs in most, but not all, of the cohort studies. These researchers and others (62) also noted that the main food source of MUFAs is animal fat, and MUFA intake is highly correlated with SFA intake and moderately correlated with TFA and PUFA intakes (56), whereby the intakes of meat, dairy, and hydrogenated vegetable oils may produce residual confounding. For instance, in 1 study (57), MUFA intake was associated with an increase in CVD risk in the highest compared with lowest quintile of intake after age-adjusted analysis (RR: 1.22; 95% CI: 0.95, 1.56). However, when adjusted for dietary and nondietary covariates, the association was attenuated (RR: 1.10; 95% CI: 0.82, 1.46), and after additional adjustment for SFAs, PUFAs, TFAs, and cholesterol, the direction of association was reversed, revealing a nonsignificant decrease in CVD risk (RR: 0.84; 95% CI: 0.53, 1.34). In the present review of controlled intervention studies that compared the effect of substituting SFA fat sources with equivalent amounts of HO oils on TC:HDL cholesterol (i.e., 10.6% of energy from SFAs replaced by 10.5% of energy from MUFAs), 7 of 8 DCs showed lower nonsignificant reductions, albeit there was a favorable weighted mean reduction of 4.0%. Interestingly, in a meta-analysis of the effects on CHD risk of consuming MUFAs in place of SFAs on the basis of changes in TC:HDL cholesterol from controlled clinical trials, researchers (60) estimated that replacing 5% of energy of SFAs with an equivalent amount of MUFAs was associated with a 7% reduction in CHD risk.
Although numerous clinical trials have examined the effect of replacing TFAs with other fats and oils on plasma lipids and lipoproteins (21), only a limited number of RCTs, consisting of 6 DCs, were identified in which the types and amounts of HO oils and TFA-containing PHVOs used in the experimental diets were adequately described (5, 18, 25, 37). In these RCTs, when TFAs from PHVOs was replaced with equivalent amounts of HO oils, all or most showed significant reductions in TC, LDL cholesterol, apoB, TGs, and TC:HDL cholesterol (P < 0.05) along with significant increases in HDL cholesterol and apoA-1 (P < 0.05). These findings are supported by a meta-analysis of 13 RCTs which showed that for each 1% of energy isocaloric replacement of TFAs with MUFAs lead to significant decreases in TC, LDL cholesterol, apoB, TGs, and TC:HDL cholesterol, along with increases in HDL cholesterol and apoA-1 (21). Similarly, in the only RCT that examined the blood lipid effects of H-OSBO in a controlled crossover diet study, substituting TFA-containing PHVOs with an equivalent amount of H-OSBO resulted in significant reductions in TC, LDL cholesterol, apoB, and TC:HDL cholesterol and an increase in apoA-1 (P < 0.05), whereas amounts of HDL cholesterol and TGs did not change (18).
Results from prospective cohort studies were consistent with the clinical trial evidence. In female nurses from the Nurses’ Health Study, replacing 2% of energy from TFAs with the same amount of unhydrogenated, unsaturated fats (i.e., MUFAs + PUFAs) was associated with a 53% lower risk of CHD, and, when replaced by an equivalent amount of MUFAs alone, a similar reduction in risk was seen (56). In a meta-analysis that estimated the effects of replacing PHVOs with other fats and oils on CHD from a pooled analysis of 4 large prospective cohort studies (21), it was estimated that replacing 7.5% of energy from PHVOs with an equivalent amount of HO sunflower oil would reduce CHD risk by 15.9%, whereas replacement with SBO produced the largest risk reduction of 21.8%.
In the present review of 11 controlled dietary trials that examined the effects of substituting HO oils for equivalent amounts of high PUFA oils that consisted primarily of LA, most showed little changes in plasma lipids and lipoproteins. In general, these findings are directionally consistent with a meta-analysis of 35 clinical trials which showed that for each 1% of energy isocaloric replacement of MUFAs with PUFAs resulted in minor reductions in HDL cholesterol, TGs, apoB, apoA-1, and TC:HDL cholesterol, whereas TC and LDL cholesterol were significantly reduced albeit to a relatively small degree (−0.015 and −0.010 mmol/L, respectively; P < 0.05) (21). In the only RCT that compared the effect of substituting H-OSBO for an equivalent amount of SBO (high in LA) on CVD risk factors (18), no significant differences in TC, LDL cholesterol, HDL cholesterol, TGs, TC:LHD cholesterol, apoB, or apoA-1 was observed after 5 wk of consuming mixed-food diets that contained 30% of energy as fat of which two-thirds was provided by the SBO test fats. Similarly, no significant changes in TC, LDL cholesterol, HDL cholesterol, and TGs was found in subjects consuming liquid formula diets for 28 d in which HO safflower oil or high-LA safflower oil served as the sole source of fat calories in 40% of energy fat diets (23). In another study the cholesterol-lowering effects of MUFAs and PUFAs were compared in a dose-response crossover design in which 4 test diets contained MUFA/PUFA amounts of 17/3% of energy, 14/6% of energy, 10/10% of energy, and 6/14% of energy respectively (74). After 6 wk on each diet, there was a progressive decrease in TC and LDL cholesterol and an increase in TGs as PUFAs replaced MUFAs, with the greatest decreases in TC and LDL cholesterol observed in diets with the highest amount of PUFAs, suggesting a greater benefit for high-PUFA diets. However, in an ANOVA analysis across the 4 diets the trend was significant for TC and TGs but was not significant for LDL cholesterol, HDL cholesterol, apoB, or apoA-1. Although no clinical trials have compared HO oils with high-PUFA oils on CHD outcomes, 1 study predicted, based on changes in TC:HDL cholesterol from short-term RCTs along with observational associations from cohort studies, that each 5% of energy replacement of SFAs with PUFAs or MUFAs would reduce occurrence of CHD by 9% and 7%, respectively (60). With the use of a similar approach that involved results from short-term RCTs, it was estimated that replacing 7.5% of energy from PHVOs with SBO or HO sunflower oil would decrease CHD risk by 8.8% and 9.2%, respectively (21).
Although little clinical trial evidence is available related to the effect of HO oils on CHD outcomes, results from the PREDIMED trial, a long-term primary prevention feeding study that spanned 4.8 y in which participants consumed a Mediterranean diet supplemented with extra-virgin olive oil, resulted in a reduced incidence of major CVD events by ∼30% compared with a control low-fat diet group (75). The Mediterranean diet contains a number of potential dietary components that may favorably affect CHD outcomes. In a systematic review of the effect of dietary factors and CHD risk (76), strong evidence from prospective cohort studies supported a link between potential protective dietary factors, including the intake of the Mediterranean diet, MUFAs, nuts, and vegetables and CHD risk, whereas evidence from clinical trials also corroborated the beneficial effects of the Mediterranean diet but not for specific dietary factors, including MUFAs, because their effect on CHD outcomes has not yet been clinically evaluated. Thus, a lack of clinical evidence remains to link the FA composition of HO oils similar to H-OSBO to CHD outcomes.
There are obvious limitations to the conclusions that can be drawn from the findings of this review related to the cardiovascular health implications of consuming H-OSBO because most of the clinical and epidemiologic results were derived from studies that evaluated HO oils other than H-OSBO. In addition, although the HO oils assessed in this review are similar in their FA composition, the level of the unsaponifiable fraction can vary substantially between different HO oil types. Major strengths include incorporating only controlled clinical trials that used HO oils substantially similar to H-OSBO (i.e., >70% oleic acid) and reasonably similar amounts of SFA, LA, and ALA (Table 1). In addition, most of the controlled diet studies that examined the blood lipid effects of HO oils compared with fats and oils high in SFAs, TFAs, or PUFAs contained isocaloric substitutions of the comparison oils and the comparison FAs, thus allowing for both an oil compared with oil and FA compared with FA assessment (15, 21–27, 29–38, 60–62, 64, 65).
In conclusion, the strength of the evidence related to the cardiovascular health implications of consuming H-OSBO suggests that the dietary replacement of fats and oils high in SFAs or TFAs with H-OSBO would favorably affect plasma lipids and lipoprotein CHD risk factors and the risk of CHD. Furthermore, the replacement of diets high in SFAs or TFAs with either H-OSBO or vegetable oils high in PUFAs (e.g., SBO, sunflower oil, corn oil) would have favorable and comparable effects on CHD risk factors and overall CHD risk.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ALA, α-linolenic acid; CHD, coronary heart disease; CVD, cardiovascular disease; DC, dietary comparison; HO, high-oleic acid; H-OSBO, high–oleic acid soybean oil; LA, linoleic acid; PHSBO, partially hydrogenated soybean oil; PHVO, partially hydrogenated vegetable oil; RCT, randomized controlled trial; SBO, soybean oil; TC, total cholesterol; TC:HDL cholesterol, ratio of total cholesterol to HDL cholesterol; TFA, trans-FA; WMC, weighted mean change.
References
- 1.Eckel RH, Borra S, Lichtenstein AH, Yin-Piazza SY; Trans Fat Conference Planning Group. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association Trans Fat Conference 2006: report of the Trans Fat Conference Planning Group. Circulation 2007;115:2231–46. [DOI] [PubMed] [Google Scholar]
- 2.Ratnayake WM, L’Abbe MR, Farnworth S, Dumais L, Gagnon C, Lampi B, Casey V, Mohottalage D, Rondeau I, Underhill L, et al. . Trans fatty acids: current contents in Canadian foods and estimated intake levels for the Canadian population. J AOAC Int 2009;92:1258–76. [PubMed] [Google Scholar]
- 3.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 2009;63(Suppl 2):S5–21. [DOI] [PubMed] [Google Scholar]
- 4.USDA and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington (DC): US Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judd JT, Clevidence BA, Muesing RA, Wittes J, Sunkin ME, Podczasy JJ. Dietary trans fatty acids: effects on plasma lipids and lipoproteins of healthy men and women. Am J Clin Nutr 1994;59:861–8. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services [Internet]. Washington (DC): The Agency. Dietary Guidelines for Americans. The Report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans, 2010. [cited 2015 Jul 29]. Available from: http://www.health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf.
- 7.Ratnayake WM, L’Abbe MR, Mozaffarian D. Nationwide product reformulations to reduce trans fatty acids in Canada: when trans fat goes out, what goes in? Eur J Clin Nutr 2009;63:808–11. [DOI] [PubMed] [Google Scholar]
- 8.USDA, Economics Research Service [Internet]. Washington (DC): The Agency; 2012 Oil Crops Yearbook. [cited 2015 Jul 29]. Available from: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1290.
- 9.USDA, Agricultural Research Service [Internet]. Washington (DC): The Agency; 2014 USDA National Nutrient Database for Standard Reference, Release 27. [cited 2015 Jul 29]. Available from: http://ndb.nal.usda.gov/ndb/search.
- 10.Institute of Shortening and Edible Oils. Food fats and oils. 9th ed. Washington (DC): The Institute; 2006 [cited 2015 Jul 29]. Available from: http://www.iseo.org/httpdocs/publications.htm.
- 11.Vistive® Gold High Oleic Soybean Oil Composition vs. Other Oils [Internet]. St Louis: Monsanto Company [cited 2015 Jul 29]. Available from: http://www.vistivegold.com/About.
- 12.Plenish High Oleic Soybean Oil Profile: Typical Fatty Acid Composition [Internet]. Wilmington (DE): DuPont [cited 2015 Jul 29]. Available from: http://www.plenish.com/food/nutritional_profile.aspx.
- 13.US Food and Drug Administration [Internet]. Food Labeling: Trans, A Rule by the Food and Drug Administration. Federal Register July 11, 2003 [accessed 2012 Apr 9; cited 2015 Jul 29]. Available from: https://www.federalregister.gov/articles/2003/07/11/03–17525/food-labeling-trans.
- 14.Health Canada [Internet]. TRANSforming the food supply: report of the Trans Fat Task Force Submitted to the Minister of Health; 2006 [cited 2015 Sep 4]. Available from: https://www.federalregister.gov/articles/2003/07/11/03-17525/food-labeling-trans.
- 15.Otite FO, Jacobson MF, Dahmubed A, Mozaffarian D. Trends in trans fatty acids reformulations of US supermarket and brand-name foods from 2007 through 2011. Prev Chronic Dis 2013;10:E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connections 2012: Soybean Market Scan, a report for United Soybean Board. November 7, 2012 [accessed on 2014 Apr 9; cited 2015 Jul 29]. Available from: http://unitedsoybean.org/wp-content/uploads/2013/07/Soybean-Market-scan-report-final.pdf.
- 17.DiRienzo MA, Astwood JD, Petersen BJ, Smith KM. Effect of substitution of low linolenic acid soybean oil for hydrogenated soybean oil on fatty acid intake. Lipids 2006;41:149–57. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr 2006;84:497–504. [DOI] [PubMed] [Google Scholar]
- 19.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011;46:209–28. [DOI] [PubMed] [Google Scholar]
- 20.Kris-Etherton PM. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation 1999;100:1253–8. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009;63(Suppl 2):S22–33. [DOI] [PubMed] [Google Scholar]
- 22.Brussaard JH, Katan MB, Groot PH, Havekes LM, Hautvast JG. Serum lipoproteins of healthy persons fed a low-fat diet or a polyunsaturated fat diet for three months. A comparison of two cholesterol-lowering diets. Atherosclerosis 1982;42:205–19. [DOI] [PubMed] [Google Scholar]
- 23.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res 1985;26:194–202. [PubMed] [Google Scholar]
- 24.Bonanome A, Grundy SM. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N Engl J Med 1988;318:1244–8. [DOI] [PubMed] [Google Scholar]
- 25.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1990;323:439–45. [DOI] [PubMed] [Google Scholar]
- 26.Wardlaw GM, Snook JT. Effect of diets high in butter, corn oil, or high-oleic acid sunflower oil on serum lipids and apolipoproteins in men. Am J Clin Nutr 1990;51:815–21. [DOI] [PubMed] [Google Scholar]
- 27.Denke MA, Grundy SM. Comparison of effects of lauric acid and palmitic acid on plasma lipids and lipoproteins. Am J Clin Nutr 1992;56:895–8. [DOI] [PubMed] [Google Scholar]
- 28.Mata P, Garrido JA, Ordovas JM, Blazquez E, Alvarez-Sala LA, Rubio MJ, Alonso R, de Oya M. Effect of dietary monounsaturated fatty acids on plasma lipoproteins and apolipoproteins in women. Am J Clin Nutr 1992;56:77–83. [DOI] [PubMed] [Google Scholar]
- 29.Ng TK, Hayes KC, DeWitt GF, Jegathesan M, Satgunasingam N, Ong A, Tan D. Dietary palmitic and oleic acids exert similar effects on serum cholesterol and lipoprotein profiles in normocholesterolemic men and women. J Am Coll Nutr 1992;11:383–90. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton PM, Derr J, Mitchell DC, Mustad VA, Russell ME, McDonnell ET, Salabsky D, Pearson TA. The role of fatty acid saturation on plasma lipids, lipoproteins, and apolipoproteins: I. Effects of whole food diets high in cocoa butter, olive oil, soybean oil, dairy butter, and milk chocolate on the plasma lipids of young men. Metabolism 1993;42:121–9. [DOI] [PubMed] [Google Scholar]
- 31.Nestel P, Clifton P, Noakes M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J Lipid Res 1994;35:656–62. [PubMed] [Google Scholar]
- 32.Zock PL, de Vries JH, Katan MB. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler Thromb 1994;14:567–75. [DOI] [PubMed] [Google Scholar]
- 33.Mata P, Alonso R, Lopez-Farre A, Ordovas JM, Lahoz C, Garces C, Caramelo C, Codoceo R, Blazquez E, de Oya M. Effect of dietary fat saturation on LDL oxidation and monocyte adhesion to human endothelial cells in vitro. Arterioscler Thromb Vasc Biol 1996;16:1347–55. [DOI] [PubMed] [Google Scholar]
- 34.Cater NB, Heller HJ, Denke MA. Comparison of the effects of medium-chain triacylglycerols, palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am J Clin Nutr 1997;65:41–5. [DOI] [PubMed] [Google Scholar]
- 35.Cuesta C, Rodenas S, Merinero MC, Rodriguez-Gil S, Sanchez-Muniz FJ. Lipoprotein profiles and serum peroxide levels of aged women consuming palmolein or oleic acid-rich sunflower oil diets. Eur J Clin Nutr 1998;52:675–83. [DOI] [PubMed] [Google Scholar]
- 36.Cater NB, Denke MA. Behenic acid is a cholesterol-raising saturated fatty acid in humans. Am J Clin Nutr 2001;73:41–4. [DOI] [PubMed] [Google Scholar]
- 37.Judd JT, Baer DJ, Clevidence BA, Kris-Etherton P, Muesing RA, Iwane M. Dietary cis and trans monounsaturated and saturated FA and plasma lipids and lipoproteins in men. Lipids 2002;37:123–31. [DOI] [PubMed] [Google Scholar]
- 38.Allman-Farinelli MA, Gomes K, Favaloro EJ, Petocz P. A diet rich in high-oleic-acid sunflower oil favorably alters low-density lipoprotein cholesterol, triglycerides, and factor VII coagulant activity. J Am Diet Assoc 2005;105:1071–9. [DOI] [PubMed] [Google Scholar]
- 39.Tholstrup T, Hjerpsted J, Raff M. Palm olein increases plasma cholesterol moderately compared with olive oil in healthy individuals. Am J Clin Nutr 2011;94:1426–32. [DOI] [PubMed] [Google Scholar]
- 40.Voon PT, Ng TK, Lee VK, Nesaretnam K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am J Clin Nutr 2011;94:1451–7. [DOI] [PubMed] [Google Scholar]
- 41.Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr 2011;105:417–27. [DOI] [PubMed] [Google Scholar]
- 42.Freidwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 43.Lin L, Allemekinders H, Dansby A, Campbell L, Durance-Tod S, Berger A, Jones PJH. Evidence of health benefits of canola oil. Nutr Rev 2013;71:370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matheson B, Walker KZ, Taylor DM, Peterkin R, Lugg D, O’Dea K. Effect on serum lipids of monounsaturated oil and margarine in the diet of an Antarctic Expedition. Am J Clin Nutr 1996;63:933–8. [DOI] [PubMed] [Google Scholar]
- 45.Noakes M, Clifton PM. Oil blends containing partially hydrogenated or interesterified fats: differential effects on plasma lipids. Am J Clin Nutr 1998;68:242–7. [DOI] [PubMed] [Google Scholar]
- 46.Palomäki A, Pohjantähti-Maaroos H, Wallenius M, Kankkunen P, Aro H, Husgafvel S, Pihlava J, Oksanen K. Effects of dietary cold-pressed turnip rapeseed oil and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome. Lipids Health Dis 2010;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Södergren E, Gustafsson IB, Basu S, Nourooz-Zadeh J, Nälsén C, Turpeinen A, Berglund L, Vessby B. A diet containing rapeseed oil-based fats does not increase lipid peroxidation in humans when compared to a diet rich in saturated fatty acids. Eur J Clin Nutr 2001;55:922–31. [DOI] [PubMed] [Google Scholar]
- 48.Uusitupa M, Schwab U, Makimattila S, Karhapaa P, Sarkkinen E, Maliranta H, Agren J, Penttila I. Effects of two high-fat diets with different fatty acid compositions on glucose and lipid metabolism in healthy young women. Am J Clin Nutr 1994;59:1310–6. [DOI] [PubMed] [Google Scholar]
- 49.Baudet MF, Jacotot B. Dietary fats and lecithin-cholesterol acyltransferase activity in healthy humans. Ann Nutr Metab 1988;32:352–9. [DOI] [PubMed] [Google Scholar]
- 50.Iggman D, Gustafsson IB, Berglund L, Vessby B, Marckmann P, Riserus U. Replacing dairy fat with rapeseed oil causes rapid improvement of hyperlipidaemia: a randomized controlled study. J Intern Med 2011;270:356–64. [DOI] [PubMed] [Google Scholar]
- 51.Karvonen HM, Tapola NS, Uusitupa MI, Sarkkinen ES. The effect of vegetable oil-based cheese on serum total and lipoprotein lipids. Eur J Clin Nutr 2002;56:1094–101. [DOI] [PubMed] [Google Scholar]
- 52.McKenney JM, Proctor JD, Wright JT Jr, Kolinski RJ, Elswick RK Jr, Coaker JS. The effect of supplemental dietary fat on plasma cholesterol levels in lovastatin-treated hypercholesterolemic patients. Pharmacotherapy 1995;15:565–72. [DOI] [PubMed] [Google Scholar]
- 53.Vega-López S, Ausman LM, Jalbert SM, Erkkilä AT, Lichtenstein AH. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr 2006;84:54–62. [DOI] [PubMed] [Google Scholar]
- 54.Hodson L, Skeaff CM, Chisholm WA. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur J Clin Nutr 2001;55:908–15. [DOI] [PubMed] [Google Scholar]
- 55.Valsta LM, Jauhiainen M, Aro A, Katan MB, Mutanen M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein levels in humans. Arterioscler Thromb 1992;12:50–7. [DOI] [PubMed] [Google Scholar]
- 56.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9. [DOI] [PubMed] [Google Scholar]
- 57.Tanasescu M, Cho E, Manson JE, Hu FB. Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am J Clin Nutr 2004;79:999–1005. [DOI] [PubMed] [Google Scholar]
- 58.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 59.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829–39. [DOI] [PubMed] [Google Scholar]
- 60.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Katarina B, Fraser FE, Goldbourt U, Hallmans G, Knekt P, Lie S, et al. . Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, Whelan J, Ramsden CE, Block RC. Fatty acids in cardiovascular health and disease: a comprehensive update. J Clin Lipidol 2012;6:216–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mensink RP, Katan MB. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med 1989;321:436–41. [DOI] [PubMed] [Google Scholar]
- 64.Mata P, Alvarez-Sala LA, Rubio MJ, Nuno J, De Oya M. Effects of long-term monounsaturated- vs polyunsaturated-enriched diets on lipoproteins in healthy men and women. Am J Clin Nutr 1992;55:846–50. [DOI] [PubMed] [Google Scholar]
- 65.Wahrburg U, Martin H, Sandkamp M, Schulte H, Assmann G. Comparative effects of a recommended lipid-lowering diet vs a diet rich in monounsaturated fatty acids on serum lipid profiles in healthy young adults. Am J Clin Nutr 1992;56:678–83. [DOI] [PubMed] [Google Scholar]
- 66.Lichtenstein AH, Ausman LM, Carrasco W, Jenner JL, Gualtieri LJ, Goldin BR, Ordovas JM, Schaefer EJ. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arterioscler Thromb 1993;13:1533–42. [DOI] [PubMed] [Google Scholar]
- 67.Carmena R, Ascaso JF, Camejo G, Varela G, Hurt-Camejo E, Ordovas JM, Martinez-Valls J, Bergstom M, Wallin B. Effect of olive and sunflower oils on low density lipoprotein level, composition, size, oxidation and interaction with arterial proteoglycans. Atherosclerosis 1996;125:243–55. [DOI] [PubMed] [Google Scholar]
- 68.Pedersen A, Baumstark MW, Marckmann P, Gylling H, Sandstrom B. An olive oil-rich diet results in higher concentrations of LDL cholesterol and a higher number of LDL subfraction particles than rapeseed oil and sunflower oil diets. J Lipid Res 2000;41:1901–11. [PubMed] [Google Scholar]
- 69.Thijssen MA, Mensink RP. Small differences in the effects of stearic acid, oleic acid, and linoleic acid on the serum lipoprotein profile of humans. Am J Clin Nutr 2005;82:510–6. [DOI] [PubMed] [Google Scholar]
- 70.Hazebroek JP. Analysis of genetically modified oils. Prog Lipid Res 2000;39:477–506. [DOI] [PubMed] [Google Scholar]
- 71.Broun P, Gettner S, Somerville C. Genetic engineering of plant lipids. Annu Rev Nutr 1999;19:197–216. [DOI] [PubMed] [Google Scholar]
- 72.Kinney AJ, Knowlton S. Designer oils: the high oleic soybean. In: Harander S, Roller S, editors. Genetic engineering for food industry: a strategy for food quality improvement. London: Blackie Academic, 1997. p. 193–213.
- 73.Hiza HAB, Bente L, Fungwe T. Nutrient Content of the U.S. Food Supply, 1909–2005: A Summary Report (Home Economics Research Report No. 58). Washington (DC): USDA, Center for Nutrition Policy and Promotion; 2008 [cited 2015 Jul 30] Available from: http://www.cnpp.usda.gov/sites/default/files/nutrient_content_of_the_us_food_supply/FoodSupply2005Report.pdf.
- 74.Howard BV, Hannah JS, Heiser CC, Jablonski KA, Paidi MC, Alarif L, Robbins DC, Howard WJ. Polyunsaturated fatty acids result in greater cholesterol lowering and less triacylglycerol elevation than do monounsaturated fatty acids in a dose-response comparison in a multiracial study group. Am J Clin Nutr 1995;62:392–402. [DOI] [PubMed] [Google Scholar]
- 75.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 76.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659–69. [DOI] [PubMed] [Google Scholar]