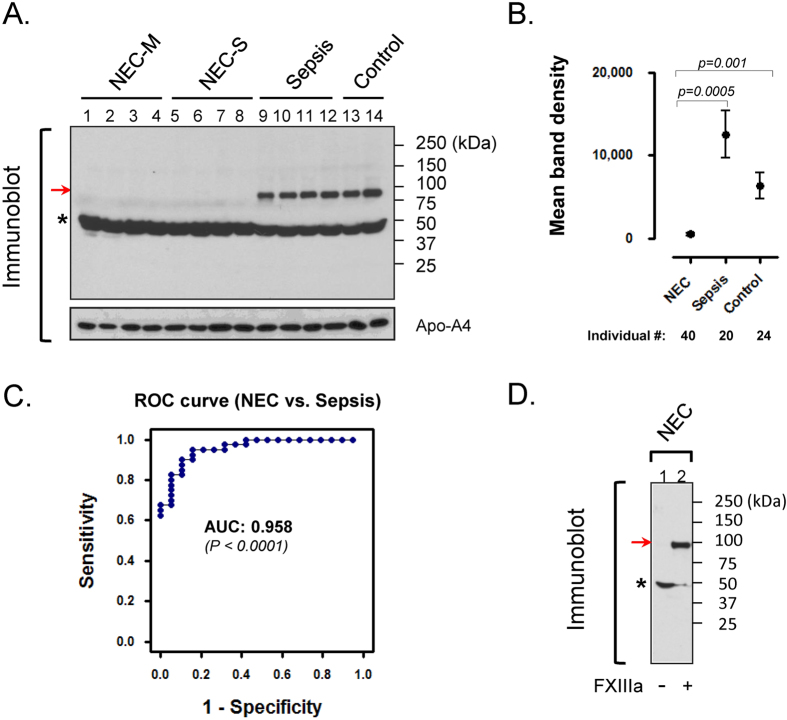
Figure 2. Lack of crosslinked FGG-dimers in plasma can efficiently differentiates NEC from neonatal sepsis.
(A) Crosslinked FGG species are absent from the plasma of infants with NEC. Equal amounts of pooled plasma (n = 5) from indicated groups were separated on SDS-PAGE, followed by immunoblot using a monoclonal antibody to FGG. Notably, FGG-monomers (indicated by asterisk) were detected in all four groups (lanes 1–14); in contrast, a FGG-dimer species (~100 kDa, indicated by arrow) was detectable only in sepsis (lanes 9–12) and control (lanes 13, 14) but not in NEC-M/S (lanes 1–8) plasmas. (B) FGG-dimer formation was individually analyzed in each sample by immunoblot using FGG antibody followed by density analysis of the identified protein bands using ImageJ software. (C) Receiver-operating characteristic (ROC) curve analysis for assessment of potential clinical utility. The data from panel-2B was statistically analyzed by ROC curve and demonstrated superior sensitivity and specificity (AUC = 0.958). (D) Addition of exogenous FXIIIa in vitro restored FGG-dimer formation in NEC samples. Pooled NEC (M/S) plasmas were incubated with (lane-2) or without (lane-1) exogenous active FXIII for 10 min. Dimer-formation was assessed by immunoblot using FGG-antibody. Note that the exogenous FXIII-induced reconstitution of FGG-dimers suggests a likely impairment of FXIII activity in NEC.