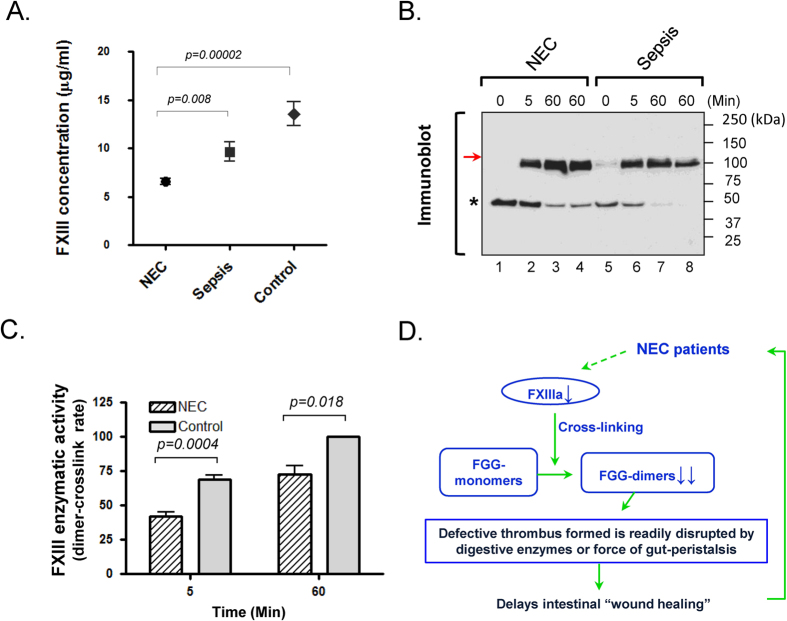
Figure 3. Absence of cross-linked FGG-dimers is correlated with insufficient enzymatic activity of FXIII in the plasma of NEC patients.
(A) Reduced plasma level of FXIII in NEC patients. Quantitative measurement was performed among the indicated groups using sandwich ELISA. Plasma levels of FXIII in NEC is significantly reduced when compared to control or sepsis cohorts. (B) Crosslinking activity of endogenous FXIII was assessed in vitro by FGG-dimer formation after the addition of FXIII activators. Pooled NEC and sepsis plasmas were incubated with the same amount of CaCl2 and thrombin (known FXIII activator) at 25 °C (lanes 1–3, 5–7) or 37 °C (lanes 4, 8) for the indicated time points. Dimer formation was detected by immunoblot using FGG-antibody. A representative result is shown from three independent experiments. (C) Reduced enzymatic activity of FXIII in NEC plasma. The protein band densities of immunoblot of panel-C were scanned, analyzed using NIH imageJ software and graphed for relative comparisons. Note that the rates of newly formed dimer vs. total (dimer + monomer) FGG bands are significantly lower in NEC compared to sepsis samples at 5 or 60 min, indicating that plasma from NEC subjects has lower enzymatic activity of FXIII than sepsis. (D) Schematic working model for the impact of reduced FXIII activity on intestinal “wound healing” in NEC patients. FXIIIa functions as a transglutaminase to covalently crosslink two molecules of FGG at the last step of the blood coagulant cascade. Without this step thrombus forms on “wounds” but is unstable, readily breaking down especially in the GI-tract given the constant exposure to both digestive enzymes and peristaltic movement. It is conceivable that this consequently delays the healing process of intestinal injuries in GI-diseases like NEC.