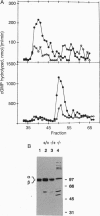
Abstract
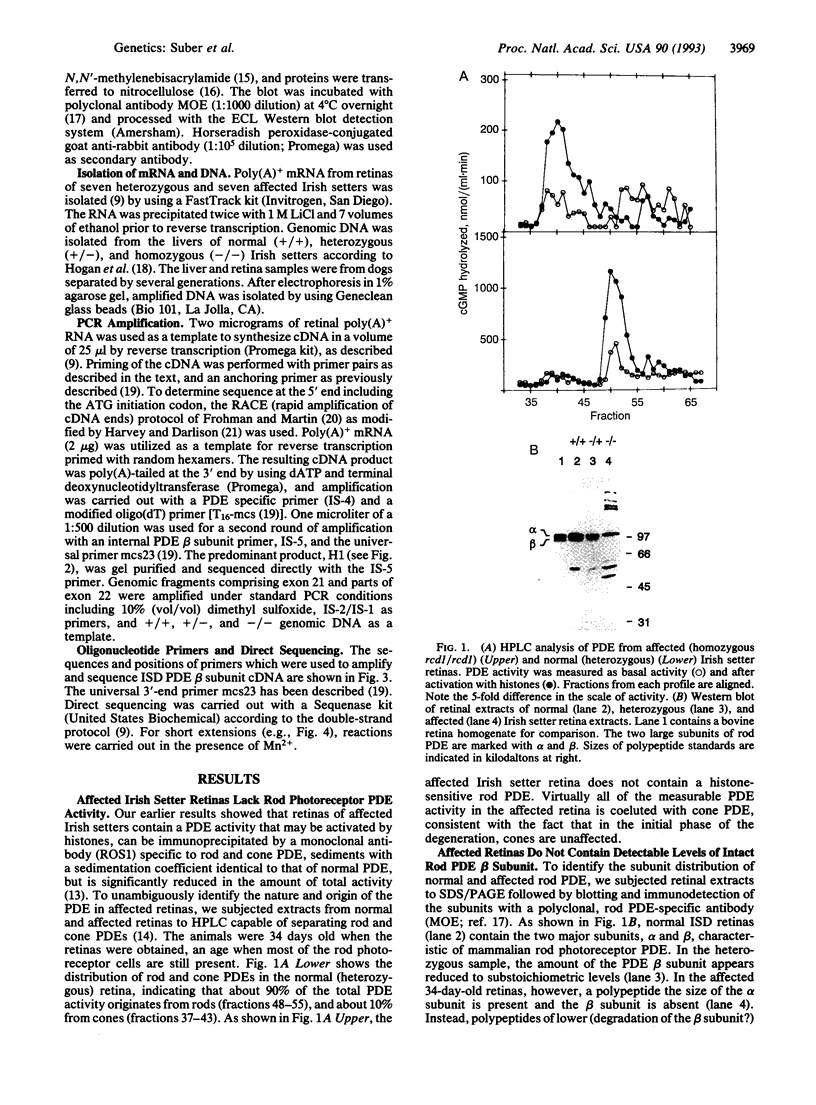
Irish setter dogs affected with a rod/cone dysplasia (locus designation, rcd1) display markedly elevated levels of retinal cGMP during postnatal development. The photoreceptor degeneration commences approximately 25 days after birth and culminates at about 1 year when the population of rods and cones is depleted. A histone-sensitive retinal cGMP phosphodiesterase (PDE; EC 3.1.4.35) activity, a marker for photoreceptor PDEs, was shown previously to be present in retinal homogenates of immature, affected Irish setters. Here we report that, as judged by HPLC separation, this activity originates exclusively from cone photoreceptors, whereas rod PDE activity is absent. An immunoreactive product the size of the PDE alpha subunit, but none the size of the beta subunit, can be detected on immunoblots of retinal extracts of affected dogs, suggesting a null mutation in the PDE beta-subunit gene. Using PCR amplification of Irish setter retinal cDNA, we determined the complete coding sequence of the PDE beta subunit in heterozygous and affected animals. The affected PDE beta-subunit mRNA contained a nonsense amber mutation at codon 807 (a G-->A transition converting TGG to TAG), which was confirmed to be present in putative exon 21 of the affected beta-subunit gene. The premature stop codon truncates the beta subunit by 49 residues, thus removing the C-terminal domain that is required for posttranslational processing and membrane association. These results suggest that the rcd1 gene encodes the rod photoreceptor PDE beta subunit and that a nonsense mutation in this gene is responsible for the production of a nonfunctional rod PDE and the photoreceptor degeneration in the rcd1/rcd1 Irish setter dogs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre G., Farber D., Lolley R., O'Brien P., Alligood J., Fletcher R. T., Chader G. Retinal degeneration in the dog. III. Abnormal cyclic nucleotide metabolism in rod-cone dysplasia. Exp Eye Res. 1982 Dec;35(6):625–642. doi: 10.1016/s0014-4835(82)80075-4. [DOI] [PubMed] [Google Scholar]
- Aguirre G. Retinal degenerations in the dog. I. Rod dysplasia. Exp Eye Res. 1978 Mar;26(3):233–253. doi: 10.1016/0014-4835(78)90072-6. [DOI] [PubMed] [Google Scholar]
- Anant J. S., Fung B. K. In vivo farnesylation of rat rhodopsin kinase. Biochem Biophys Res Commun. 1992 Mar 16;183(2):468–473. doi: 10.1016/0006-291x(92)90505-f. [DOI] [PubMed] [Google Scholar]
- Anant J. S., Ong O. C., Xie H. Y., Clarke S., O'Brien P. J., Fung B. K. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992 Jan 15;267(2):687–690. [PubMed] [Google Scholar]
- Aquirre G., Farber D., Lolley R., Fletcher R. T., Chader G. J. Rod-cone dysplasia in Irish setters: a defect in cyclic GMP metabolism in visual cells. Science. 1978 Sep 22;201(4361):1133–1134. doi: 10.1126/science.210508. [DOI] [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Catty P., Deterre P. Activation and solubilization of the retinal cGMP-specific phosphodiesterase by limited proteolysis. Role of the C-terminal domain of the beta-subunit. Eur J Biochem. 1991 Jul 15;199(2):263–269. doi: 10.1111/j.1432-1033.1991.tb16119.x. [DOI] [PubMed] [Google Scholar]
- Chader G. J., Fletcher R. T., Sanyal S., Aguirre G. D. A review of the role of cyclic GMP in neurological mutants with photoreceptor dysplasia. Curr Eye Res. 1985 Jul;4(7):811–819. doi: 10.3109/02713688509020039. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber D. B., Danciger J. S., Aguirre G. The beta subunit of cyclic GMP phosphodiesterase mRNA is deficient in canine rod-cone dysplasia 1. Neuron. 1992 Aug;9(2):349–356. doi: 10.1016/0896-6273(92)90173-b. [DOI] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Darlison M. G. Random-primed cDNA synthesis facilitates the isolation of multiple 5'-cDNA ends by RACE. Nucleic Acids Res. 1991 Jul 25;19(14):4002–4002. doi: 10.1093/nar/19.14.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz R. L., Bunt-Milam A. H., Chang M. L., Beavo J. A. cGMP phosphodiesterase in rod and cone outer segments of the retina. J Biol Chem. 1985 Jan 10;260(1):568–573. [PubMed] [Google Scholar]
- Inglehearn C. F., Bashir R., Lester D. H., Jay M., Bird A. C., Bhattacharya S. S. A 3-bp deletion in the rhodopsin gene in a family with autosomal dominant retinitis pigmentosa. Am J Hum Genet. 1991 Jan;48(1):26–30. [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K., Hahn L. B., Mukai S., Travis G. H., Berson E. L., Dryja T. P. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Lieberman B. S., Hurwitz R. L., Lolley R. N. Phosphodiesterase-probes show distinct defects in rd mice and Irish setter dog disorders. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1569–1579. [PubMed] [Google Scholar]
- Liu Y. P., Krishna G., Aguirre G., Chader G. J. Involvement of cyclic GMP phosphodiesterase activator in an hereditary retinal degeneration. Nature. 1979 Jul 5;280(5717):62–64. doi: 10.1038/280062a0. [DOI] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- PARRY H. B. Degenerations of the dog retina. II. Generalized progressive atrophy of hereditary origin. Br J Ophthalmol. 1953 Aug;37(8):487–502. doi: 10.1136/bjo.37.8.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. The molecular genetics of retinal photoreceptor proteins involved in cGMP metabolism. Prog Clin Biol Res. 1991;362:33–66. [PubMed] [Google Scholar]
- Qin N., Pittler S. J., Baehr W. In vitro isoprenylation and membrane association of mouse rod photoreceptor cGMP phosphodiesterase alpha and beta subunits expressed in bacteria. J Biol Chem. 1992 Apr 25;267(12):8458–8463. [PubMed] [Google Scholar]
- Rosenfeld P. J., Cowley G. S., McGee T. L., Sandberg M. A., Berson E. L., Dryja T. P. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet. 1992 Jun;1(3):209–213. doi: 10.1038/ng0692-209. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Brown M. S., Slaughter C. A., Südhof T. C., Goldstein J. L. Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell. 1992 Sep 18;70(6):1049–1057. doi: 10.1016/0092-8674(92)90253-9. [DOI] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Riess O., Hutchinson G., Collins C., Lin B. Y., Kowbel D., Andrew S., Schappert K., Hayden M. R. Genomic organization and complete sequence of the human gene encoding the beta-subunit of the cGMP phosphodiesterase and its localisation to 4p 16.3. Nucleic Acids Res. 1991 Nov 25;19(22):6263–6268. doi: 10.1093/nar/19.22.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Ubaidi M. R., Pittler S. J., Champagne M. S., Triantafyllos J. T., McGinnis J. F., Baehr W. Mouse opsin. Gene structure and molecular basis of multiple transcripts. J Biol Chem. 1990 Nov 25;265(33):20563–20569. [PubMed] [Google Scholar]