Abstract
The misfolding and aggregation of the Aβ peptide – a fundamental event in the pathogenesis of Alzheimer’s disease – can be instigated in the brains of experimental animals by the intracranial infusion of brain extracts that are rich in aggregated Aβ. Recent experiments have found that the peripheral (intraperitoneal) injection of Aβ seeds induces Aβ deposition in the brains of APP-transgenic mice, largely in the form of cerebral amyloid angiopathy. Macrophage-type cells normally are involved in pathogen neutralization and antigen presentation, but under some circumstances, circulating monocytes have been found to act as vectors for the transport of pathogenic agents such as viruses and prions. The present study assessed the ability of peripheral monocytes to transport Aβ aggregates from the peritoneal cavity to the brain. Our initial experiments showed that intravenously delivered macrophages that had previously ingested fluorescent nanobeads as tracers migrate primarily to peripheral organs such as spleen and liver, but that a small number also reach the brain parenchyma. We next injected CD45.1-expressing monocytes from donor mice intravenously into CD45.2-expressing host mice; after 24 hours, analysis by fluorescence-activated cell sorting (FACS) and histology confirmed that some CD45.1 monocytes enter the brain, particularly in the superficial cortex and around blood vessels. When the donor monocytes are first exposed to Aβ-rich brain extracts from human AD cases, a subset of intravenously delivered Aβ-containing cells migrate to the brain. These experiments indicate that, in mouse models, circulating monocytes are potential vectors by which exogenously delivered, aggregated Aβ travels from periphery to brain, and more generally support the hypothesis that macrophage-type cells can participate in the dissemination of proteopathic seeds.
Keywords: Aβ, Alzheimer’s disease, amyloid, cerebral amyloid angiopathy, macrophage, prion
1. Introduction
The aggregation of the beta-amyloid peptide (Aβ) in the brain is an early and integral event in the pathogenesis of Alzheimer’s disease (Hardy and Selkoe, 2002; Holtzman et al., 2011). Aβ normally exists in a structurally unfolded (intrinsically disordered) state, but in its pathogenic form, Aβ becomes rich in β-sheet and induces the misfolding and subsequent self-assembly of other Aβ molecules. These durable, prion-like multimeric seeds instigate the formation of senile plaques and cerebral β-amyloid angiopathy as monomeric Aβ is recruited into the β-sheet-rich deposits (Jucker and Walker, 2013; Walker et al., 2006; Walker and LeVine, 2012). We and others have found that intracranial injections of Aβ multimers seed Alzheimer-like pathology in Aβ-precursor protein (APP) transgenic rodent models (Duran-Aniotz et al., 2014; Eisele et al., 2009; Fritschi et al., 2014; Hamaguchi et al., 2012; Kane et al., 2000; Langer et al., 2011; Meyer-Luehmann et al., 2006; Morales et al., 2012a; Morales et al., 2012b; Rosen et al., 2012; Stohr et al., 2012; Stohr et al., 2014; Watts et al., 2011; Watts et al., 2014).
Within the brain, the proteinaceous lesions that characterize Alzheimer’s disease and other protein misfolding disorders appear to propagate among interconnected brain areas, suggestive of the dissemination of seeds by means of axonal transport (Boluda et al., 2015; Clavaguera et al., 2009; Clavaguera et al., 2014; Guo and Lee, 2011; Guo and Lee, 2014; Hyman, 2014; Liu et al., 2012; Walker and LeVine, 2012; Walker et al., 2013; Ye et al., 2015). Other studies have shown that the injection of Aβ multimers into the peritoneal cavity of APP-transgenic mice can seed Aβ deposition in the brain, particularly in the form of cerebral β-amyloid angiopathy (Eisele et al., 2010; Eisele et al., 2014). While the evidence currently favors axonal transport as a key mode of lesion propagation within the nervous system, the mechanisms by which Aβ seeds are transported from periphery to brain remain uncertain. The preponderance of amyloid angiopathy in the forebrain of intraperitoneally seeded APP23 transgenic mice (Eisele et al., 2010) suggests the possibility that the seeds reach the brain via the vasculature (Eisele et al., 2014). Furthermore, the presence of Aβ within circulating monocytes of these mice implicates these cells as possible vectors for the transport of seeds from periphery to brain (Eisele et al., 2014). The poor ability of microglia to degrade amyloid fibrils further supports the idea that aggregated Aβ may remain intact in macrophages for a long period of time (Frackowiak et al., 1992), but the evidence for the entry of Aβ-laden macrophages from the circulation into the brain remains indirect.
As a component of the innate immune system, macrophages normally serve to phagocytose and degrade exogenous pathogens such as microbes, and they also present antigen to cells of the adaptive immune system (Alberts et al., 2002). However, in some instances macrophages have been found to ingest and disseminate pathogens intact (Ferreira et al., 2010; Johnson et al., 2010; Kirby et al., 2009; Tanaka et al., 2012), thereby contributing to the disease process. In the brain, microglia are resident macrophages that originate from the yolk sac early in embryogenesis and replenish themselves by self-replication (Prinz et al., 2011). Particularly in disease states, circulating (hematogenous) monocytes can differentiate into macrophages in the brain, where they become part of local cellular networks (Mildner et al., 2007; Priller et al., 2001). Studies in which the blood-brain barrier is disrupted by irradiation demonstrate an increased infiltration of circulating monocytes into the brain (Mildner et al., 2007). Furthermore, disease models of multiple sclerosis show that hematogenous monocytes enter the brain and are responsible for stripping myelin from axons (Lampert, 1978). Activation of circulating monocytes in an Alzheimer’s disease model resulted in the increased phagocytosis of cerebral β-amyloid, thereby reducing the number of senile plaques (Shaftel et al., 2007; Town et al., 2008). Thus, in disease states it is clear that circulating monocytes are able to gain access to the brain, but it is thought that few, if any, circulating monocytes cross the intact blood-brain barrier to enter the healthy brain (Kroll and Neuwelt, 1998; Prinz et al., 2011; Zhang and Pardridge, 2001). In the present study, we tested the hypothesis that monocytes are able to phagocytose and convey cargo from the peritoneal cavity to the brain in healthy mice. We found that limited numbers of these cells can enter the brain parenchyma, and thus could act as vectors for the transport of proteopathic seeds.
2. Results
2.1. Characterization of lavage cell-types by FACS
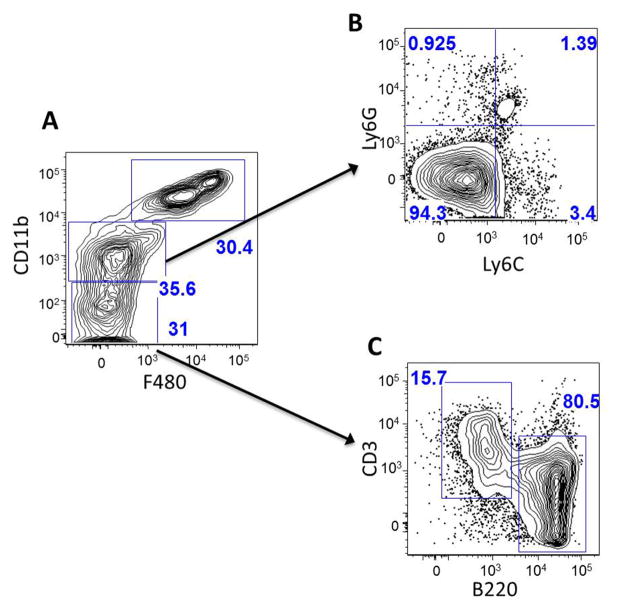
To characterize the cells collected by lavage, FACS analysis was performed using antibodies specific for macrophages and lymphocytes (Figure 1). The analysis showed 3 distinct populations based on the expression of specific markers: CDllb-high/F4/80-high; CDllb-intermediate/F4/80-negative; and CDllb-negative/F4/80-negative. Roughly 30 percent of the lavage consisted of large peritoneal macrophages (LPM), which are characterized by high expression of CDllb and F4/80 (Ghosn et al., 2010). The cell population characterized by intermediate CDllb and negative F4/80 expression was gated and investigated for Ly6G and Ly6C expression. The majority (94.3 percent) of the cells were Ly6G-negative and Ly6C-negative, indicating that these cells are small peritoneal macrophages (Ghosn et al., 2010; Gordon and Taylor, 2005; Rose et al., 2012). The CDllb-negative/F4/80negative cells were gated and investigated for expression of B220 (B-cells) and CD3 (T-cells) (Rodig et al., 2005). Of this subpopulation, 80.5 percent of the cells were B-cells and 15.7 percent were T-cells. Overall, this analysis indicates that the lavage cells are ~60% macrophages (LPM and SPM) and ~30% lymphocytes (most of which are B-cells).
Fig. 1. Characterization of lavaged leukocytes by FACS analysis.
A. Whole lavage sample. Upper right gate (30.4%) represents CDllb-high & F4/80-high cells, which are large peritoneal macrophages (LPM). The middle gate (35.6%) represents CDllb-intermediate and F4/80-negative cells. This fraction of cells was further probed for expression of Ly6G and Ly6C (B). 94.3% of these cells were Ly6G-negative and Ly6C-negative, indicating that they are small peritoneal macrophages (SPM). The bottom gate of panel A denotes CDllb-negative and F4/80-negative cells. This fraction was further probed for markers of B-cells (B220) and T-cells (CD3) (C). 80.5 percent of these cells were B-cells and 15.7% were T-cells. Overall, this analysis indicates that the lavage cells consist of ~60% macrophages (LPM and SPM), ~30% lymphocytes (most of which are B-cells), and 10% other cells.
2.2. Systemic distribution of labeled macrophages
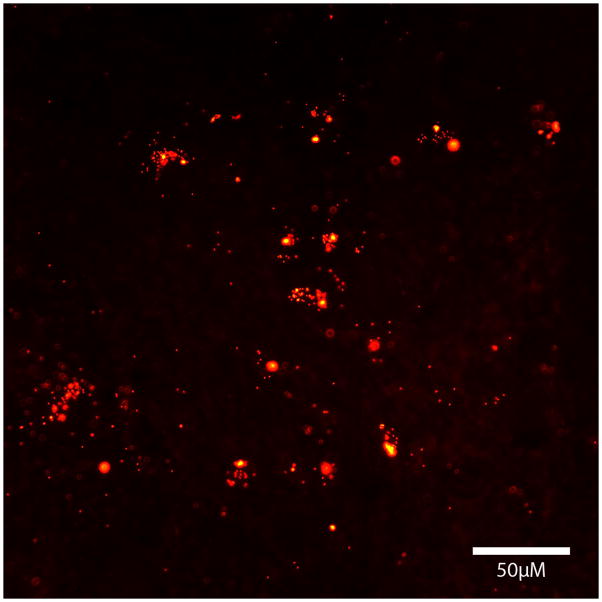
To determine the general distribution of macrophages that had previously ingested fluorescent nanobeads, we assessed nanobead-labeled cells histologically in the brain and systemic organs. Host mice received either i.p. injections of nanobeads (ingested by endogenous macrophages), or i.v. injections of exogenous, nanobead-laden macrophages harvested from the peritoneal cavity of donor mice. In both groups, the systemic distribution of macrophages was similar, i.e., both endogenous and exogenous macrophages had comparable patterns of distribution in the body. Our analysis then focused on the mice receiving exogenous macrophages. At both four days and four weeks post-infusion of cells, bead-laden phagocytes were primarily found in the spleen (Figure 2), liver, and kidney of the host mice, and (in much smaller quantities) in lung and blood (not shown). The spleen in particular contained more nanobeads than any other tissue, consistent with its role in the storage and deployment of monocytes (Swirski et al., 2009). In addition, a small number of bead-containing macrophages (<50 per 40μm section at 4 days and <10 per 40μm section at 4 weeks) were detected in grey matter structures and superficial cortex of the brain (not pictured).
Fig. 2.
Fluorescent nanobeads (red) in the spleen of a host mouse 4 weeks after i.v. injection of bead-laden macrophages from a donor mouse.
2.3. A limited number of exogenous macrophages enter the intact brain
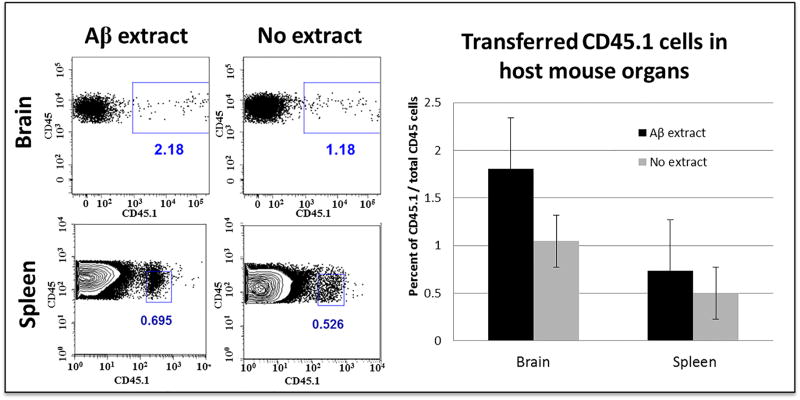
To quantify more precisely the exogenous macrophages that enter the intact brain in our paradigm, macrophages were elicited in the peritoneal cavity of CD45.1-expressing donor mice and then infused i.v. into CD45.2-expressing host mice. Twenty-four hours later, the host brains were gently homogenized and the blood vessels depleted from the homogenates to eliminate cells that might have remained within the vascular lumen following perfusion. For comparison, we also assessed the population of exogenous macrophages that had migrated to the spleen at the same time point. A small number of donor macrophages were detectable in the brain parenchyma 24 hours after i.v. infusion, and, as expected, many more donor cells had migrated to the spleen (Figure 3).
Fig. 3.
FACS analysis of intravenously transferred CD45.1 cells in the brain and spleen of a B6[CD45.2] host mouse. Cells were gated for CD45 immunoreactivity (all hematopoietic cells) and CD45.1 (exogenous donor cells). Exposure of the donor cells to Aβ-rich AD brain extract in the i.p. injectate did not significantly influence the relative quantity of transferred cells that entered the brain or spleen.
2.4. Exogenous macrophages containing Aβ seeds enter the intact brain
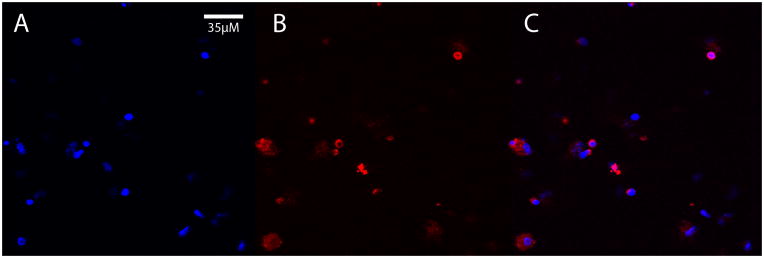
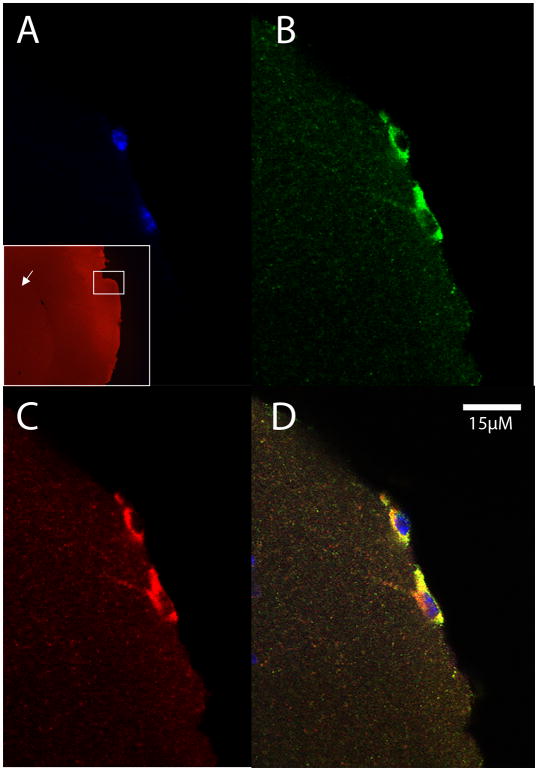
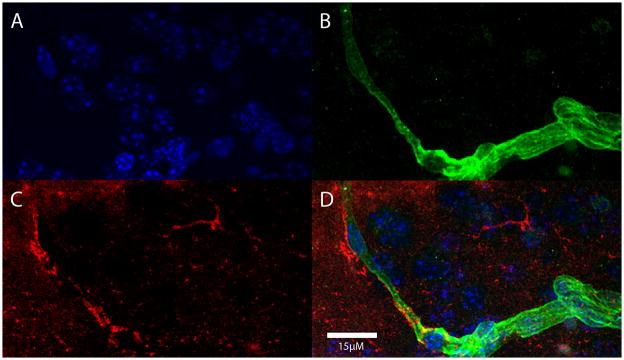
Having established that macrophages can enter the brain from the bloodstream, even after ingestion of nanobeads, we next asked whether exogenous macrophages that had ingested Aβ-rich brain extract also can enter the brain. First, to confirm the ingestion of human Aβ by peritoneally elicited macrophages, wild-type mice received i.p. injections of AD brain extract containing ample aggregated Aβ, and after 4 days the cells were harvested, plated on coverslips, and immunostained with antibody 6E10 to human-sequence Aβ, which does not recognize murine Aβ (K.S. et al., 1988). Confocal microscopy verified that macrophages ingest detectable quantities of the Aβ-laden brain extract (Figure 4). CD45.1 macrophages containing human Aβ were then infused i.v. into B6[CD45.2] host mice; after a 24 hour incubation period, immunohistochemistry revealed Aβ-containing cells in the superficial neocortex and subarachnoid space (Figure 5). Additionally, some Aβ-immunoreactive cells were localized around blood vessels (Figure 6), but we did not detect CD45.1 in these perivascular cells. The reason for the absence of the donor marker in perivascular cells is not clear, but suggests either that CD45.1 was undetectable or that the Aβ may have been transferred from exogenous donor cells to host macrophages.
Fig. 4.
Macrophages harvested from the peritoneal cavity following i.p. injection of AD brain extract contain immunodetectable human Aβ. A) Cell nuclei stained with DAPI (blue); B) Aβ immunofluorescence (red, antibody 6E10); C) merged images.
Fig. 5.
Exogenous macrophages from a CD45.1-expressing donor mouse in the superficial neocortex of a CD45.2-expressing host mouse. The cells were exposed to Aβ-rich AD brain extract in the peritoneal cavity of donor mice, collected by lavage, and injected intravenously into the host mouse 24 hours prior to sacrifice. A) DAPI nuclear stain (blue) with low magnification inset showing the location of the cells (white box) in the superficial neocortex (arrow marks the striatum); B) CD45.1 (green); C) Aβ (red); D) merged images.
Fig. 6.
Aβ immunoreactivity (red) adjacent to a laminin-immunoreactive blood vessel (green) in the neocortex of a CD45.2-expressing host mouse 24 hours after an i.v. infusion of exogenous macrophages. In this case, the Aβ-immunoreactive cells were not demonstrably immunopositive for the donor antigen (CD45.1). A) DAPI nuclear stain (blue); B) laminin (green); C) Aβ (red); D) merged images.
3. Discussion
Both circulating monocytes and microglial cells are, under the appropriate circumstances, involved in the immune response in the brain. These two types of innate immune cells are among the first to respond to pathological insults; they help to repair physical trauma, defend against pathogens, and remove debris such as the remnants of dead cells (Flannagan et al., 2009; Gregory and Devitt, 2004). While they share some common functions, monocytes and microglia have distinct origins (Epelman et al., 2014; Gautier et al., 2012; Hettinger et al., 2013; Murray et al., 2014) and they monitor different tissue domains within the brain. When alerted to a pathological event in the CNS, the resident microglia are responsible for initiating the immune response, which can include the recruitment of circulating monocytes (Gate et al., 2010). The role of peripheral monocytes that enter the brain remains somewhat ambiguous, in part because they can be difficult to distinguish from resident microglia (Prinz et al., 2011).
Circulating monocytes enter the brain under various pathological conditions, including immunosuppression (Bauer et al., 1995) and irradiation injury (Mildner et al., 2007). In addition, monocytes enter the brain when the blood-brain barrier is physically compromised, for example in cases of stroke (Schilling et al., 2003; Tanaka et al., 2003) or head injury (Szmydynger-Chodobska et al., 2012). Our study indicates that a small but consistent subset of exogenous circulating macrophages enter the intact, healthy brain, and this was so whether they contained nanobeads, Aβ-rich brain extract, or no specific cargo.
Histological evidence that hematogenous monocytes can enter the brains of healthy mice was confirmed by FACS analysis. The brain samples for FACS underwent perfusion and vascular depletion to minimize the possibility that the exogenous cells detected by the cell sorter were within the blood vessels of the brain, rather than on the parenchymal (abluminal) side of the blood-brain barrier (Triguero et al., 1990). However, we cannot rule out the possibility that some of these cells were within the vasculature, for example as dislodged marginating cells (van Furth and Sluiter, 1986), or in areas lacking capillary endothelial tight junctions such as the circumventricular organs or choroid plexus. However, in our histological samples we did not detect exogenous cells in these regions, whereas they were evident in the brain parenchyma, indicating that some circulating cells do cross from blood to brain.
FACS analysis of cell types within the lavage sample demonstrated the presence of both macrophages (60%) and lymphocytes (30%). We believe that the macrophages are most likely to be responsible for the transport and dissemination of Aβ seeds because they are the principal cell type that is specialized for the removal of dying/dead cells and cellular debris (Perry et al., 2010). Some evidence suggests that B-cells can be phagocytic in various disease states (Borrello and Phipps, 1996), and thus could contribute to the transport of Aβ seeds. In contrast to seed dissemination, the presence of lymphocytes (which were primarily B-cells in the lavage sample) could result in the presentation of antigens to Aβ. Macrophages also present antigen (Unanue, 1984), so it is important to consider the possibility that engagement of the adaptive immune response could eventually result in antibody production and a decrease in cerebral Aβ deposition. Long-term studies of the impact of exogenous, Aβ-containing immune cells will be needed to address this question.
Taken together, our studies indicate that macrophages are able to take up and transport material from the periphery to the brain through the vasculature. In this light, the findings support the hypothesis that peripheral macrophages are a source of amyloidogenic Aβ seeds that traffic to the brain from the peritoneal cavity in an experimental seeding model (Eisele et al., 2010; Eisele et al., 2014). The localization of cells adjacent to cerebral blood vessels further supports this conclusion, in that the Aβ that deposits in the brains of peripherally seeded APP23 mice is mainly in the form of amyloid angiopathy (Eisele et al., 2010; Eisele et al., 2014).
In humans, there is currently no evidence that Alzheimer’s disease can be induced by peripheral seeds (Irwin et al., 2013; Jucker and Walker, 2013). However, the fundamental molecular mechanisms by which proteopathic seeds arise and spread are unlikely to differ among species. At the cellular level, the role of macrophages – both central and peripheral – in the deposition of Aβ remains uncertain (Gate et al., 2010). Some studies have indicated that these cells can reduce the amount of Aβ in the brain (Simard et al., 2006), whereas others indicate that they promote deposition (Akiyama et al., 2000). It is possible that both processes are at play, depending on the circumstances. For example, the removal of senile plaques in Alzheimer patients who had been immunized against Aβ was associated with an increase in the amount of cerebral Aβ angiopathy (Boche et al., 2008; Ferrer et al., 2004; Masliah et al., 2005; Nicoll et al., 2003), suggesting that Aβ seeds were taken up and transported from the plaques to the vascular wall by macrophages (Boche et al., 2008; Masliah et al., 2005). Our model supports a general role of macrophages in the uptake and dissemination of Aβ seeds; how the seeds exit the cells and stimulate Aβ deposition in the vascular wall remains to be determined.
Another potential mechanism by which proteopathic seeds might reach the brain is axonal transport. In a preliminary experiment, we found that the intraperitoneal injection of the tracer fluorogold led to retrogradely labeled cells only in the dorsal motor nucleus of the vagus nerve (data not shown), an area that does not exhibit aggregated Aβ in peripherally seeded models. Even so, we cannot yet rule out a role of neuronal transport in the translocation of Aβ seeds from periphery to brain. Prions, for example, can reach the CNS from the periphery by neuronal transport (Bartz et al., 2005; McBride et al., 2001; Sigurdson et al., 2001; van Keulen et al., 1999). Recent experiments also indicate that α-synuclein seeds are transported by the vagus nerve from the intestinal wall to the brain (Holmqvist et al., 2014). Within the CNS, cellular uptake and axonal transport mechanisms are implicated in the systematic spread of a number of pathogenic protein aggregates, including prions (Aguzzi, 2003; Borchelt et al., 1994; Buyukmihci et al., 1983; Liberski et al., 1990; Liberski et al., 2012; Scott et al., 1992), Aβ (Hallbeck et al., 2013; Hamaguchi et al., 2012; Lee et al., 2010; Saper et al., 1987; Ye et al., 2015), tau (Braak and Del Tredici, 2011; Clavaguera et al., 2009; Holmes et al., 2014) and α-synuclein (Angot et al., 2012; Desplats et al., 2009; Luk et al., 2012; Masuda-Suzukake et al., 2014).
In summary, our findings support the hypothesis that phagocytic cells are able to transport ingested cargo – including aggregated Aβ – from the periphery to the brain in a mouse model. Macrophages thus appear to play contradictory roles in Aβ deposition. On the one hand, they are predisposed to remove foreign substances and present antigen, but if they are unable to fully degrade the material, they may inadvertently disseminate it to other parts of the body, including the brain. By clarifying the involvement of macrophages in the trafficking of proteopathic seeds, we hope to pinpoint key mechanisms that can be targeted to slow this process within the brain.
4. Experimental procedures
4.1 Subjects
Non-transgenic C57BL/6 mice served as subjects. For the FACS/histology experiments, wild-type, inbred C57BL/6 mice expressing the hematopoietic cell marker CD45.2 (B6[CD45.2] mice) served as hosts, and congenic B6[CD45.1] mice (B6.SJL-Ptprca Pepcb/BoyJ; The Jackson Laboratory) served as donors. CD45 is a pan-leukocytic antigen that has two differentiable allelic forms in the two murine lines; as such, CD45.1-expressing cells can be readily distinguished from CD45.2-expressing cells. Mice were housed in small groups under standard conditions at a temperature of 22°C and a 12 h light/dark cycle with ad libitum access to food and water. All experimental procedures were carried out in accordance with US federal guidelines, and were approved by the Emory Institutional Animal Care and Use Committee (IACUC).
4.2. Characterization of lavaged leukocytes by fluorescence-activated cell sorting (FACS)
To characterize the cell population that was induced in the mice, C57/BL6 mice received a 100μl intraperitoneal injection of thioglycolate on day 0, and intraperitoneal cells were collected by lavage on day 4 and gently centrifuged at 1000 x g for 5 min (Ray and Dittel, 2010). The supernatant was removed, the red blood cells lysed in diH2O, and the leukocytic fraction was re-sedimented at 1000 x g for 5 min and re-suspended in buffered physiological saline. This fraction was incubated with antibodies to CD11b and F4/80 (macrophage-specific antigens), Ly6G and Ly6C (transiently expressed on monocytes in the bone marrow), B220 (a pan B-cell marker) and CD3 (primarily expressed by T-cells) for 25 minutes on ice in CD16/CD32 mouse BN Fc Block (BD Pharmingen) (chromogens FITC and phycoerythrin [PE]). After washing, fixing buffer was added and the pellet was washed again. The pellet was resuspended in FACS buffer (PBS+ 2% FBS) and analyzed by FACS for expression of CD11b and F4/80 on double-positive cells. The cells were gated by size (forward scatter) and density (side scatter), and then plotted according to CD11b and F4/80 expression. The resulting population was further gated by level of expression. CD11b-intermediate and F4/80-negative expressing cells were gated and plotted according to Ly6G and Ly6C expression. CD11b-negative and F4/80-negative expressing cells were gated and plotted according to B220 and CD3 expression. In this way, the composition of cells in the lavage fraction can be quantified (see Results).
4.3. Trafficking of macrophages bearing fluorescent nanobeads
Our first objective was to assess the systemic distribution of macrophages that had ingested fluorescent nanobeads as tracer cargo. Five wild-type C57/BL6 mice received intraperitoneal (i.p.) injections of red fluorescent nanobeads (SPHERO™ Fluorescent Nile Red Particles; 2 μl of 1% nanobeads [w/v], 0.53μm diameter, Spherotech, Lake Forest, IL, USA) along with 100μl of thioglycolate to elicit macrophages on day 0. On day 3, mice were given a second injection of nanobeads, and animals were then sacrificed on day 4. In one group of mice (n=5), the nanobead-containing macrophages were analyzed in the mice that received the nanobead injections, i.e., the tracked macrophages originated endogenously within the same mouse. In a separate group of mice (n=10), bead-laden macrophages were prepared in donor mice as described above, and then harvested and infused intravenously (i.v.) into host mice (exogenous macrophages).
Leukocytes were collected by lavage (Ray and Dittel, 2010) and gently centrifuged at 1000 x g for 5 min. The supernatant was removed, the red blood cells lysed in diH2O, and the leukocytic fraction re-sedimented at 1000 x g for 5 min, re-suspended in buffered physiological saline, and cells counted using a hemocytometer. The cells were assessed for viability in a test sample by trypan blue exclusion. In all cases, a minimum of 75% of the cells for infusion were viable. In each host mouse, 5×106 viable cells in 250μl physiological saline were slowly infused intravenously via the tail vein. Following an incubation period of either 4 days or 4 weeks, the host mice were perfused with 200mL of cool (4–8°C) physiological saline and the following tissues collected: Brain, spleen, liver, lung, heart, kidney, pancreas, and blood.
4.4. FACS analysis of CD45.1-expressing exogenous macrophages transferred to B6[CD45.2] host mice
Our second objective was to determine the proportion of exogenous macrophages that enter the brain from the bloodstream following infusion of the cells into host mice. A total of 15 mice served as hosts in two separate experimental runs. Macrophages were elicited in the peritoneal cavity of B6[CD45.1] donor mice by i.p. injection of thioglycolate on Day 1 and Day 3, and the cells were collected by lavage on day 4 (Ray and Dittel, 2010), as described above. The CD45.1-bearing cells (5×106 cells/250μl) were then slowly infused i.v. into CD45.2-expressing host mice. 24 hours later, the host mice were transcardially perfused with 200mL of cool (4–8°C) physiological saline under deep Nembutal anesthesia. The whole brain and spleen were separately processed and the blood vessels depleted from the homogenates (D’Alessandro et al., 2013; Pertoft, 2000) as follows: The organs were homogenized over a 100μm mesh nylon sieve, lysed in collagenase IV (Worthington), and mononuclear cells (along with a small population (~10%) of other leukocytes; see Figure 1) were obtained by density-gradient centrifugation using Percoll (Amersham) solutions (40% and 70%). Cells were incubated with antibodies to CD45 (a pan-hematopoietic cell antigen) and CD45.1 for 25 minutes on ice in CD16/CD32 mouse BN Fc Block (BD Pharmingen) (chromogens FITC and phycoerythrin [PE]). After washing, fixing buffer was added and the pellet was washed again. The pellet was resuspended in FACS buffer (PBS + 2% FBS) and analyzed by FACS for expression of CD45.1 on CD11b/CD45 double-positive cells. The cells were gated by size (forward scatter) and density (side scatter), and then plotted according to CD45 and CD45.1 expression. In this way, the entry of exogenous macrophages from the donor mouse can be assessed quantitatively in the brain and spleen of the host mouse.
To further characterize the cells and tissues in this experiment, induced intraperitoneal cells were harvested from donor mice on day 4 and plated in EMEM (Eagle’s Minimum Essential Medium). To promote growth we used 10ng/ml of macrophage colony-stimulating factor (M-CSF). Murine macrophages were isolated according to the protocol of Fortier and Falk (Fortier and Falk, 2001). The cells were maintained for 3 days before they were dried on slides and processed for immunocytochemistry. For histology, the brains of B6[CD45.2] host mice were sectioned at 40μm thickness (as described below) and stained with antibodies 6E10 to human Aβ, laminin (blood vessels), and CD45.1 (transferred hematopoietic cells (see section 4.6 below for antibody details). Tissues were counterstained with a fluorescent nuclear marker (4′,6-diamidino-2-phenylindole [DAPI]).
4.5. Trafficking of macrophages bearing Aβ-rich brain extract
As a third objective, we sought to assess the fate of macrophages that had specifically ingested Aβ-rich brain extract to determine whether these cells can subsequently enter the host brain. The extract was prepared as described previously (Kane et al., 2000; Meyer-Luehmann et al., 2006). In brief, neocortical tissue from a histopathologically verified AD case was homogenized at 10% (w/v) in PBS at 4°C, followed by brief sonication, also at 4°C. The homogenate was centrifuged at 3,000×g for 5 minutes at 4°C, and the supernatant was aliquoted and immediately frozen at −80°C until use. Donor B6[CD45.1] mice received i.p. injections of either thioglycolate alone (resulting cells infused into 6 host mice) or thioglycolate and 250μl of 10% AD brain extract (resulting cells infused into 9 host mice) on day 0 and day 3, and were sacrificed on day 4. Macrophages were collected by lavage, processed, counted, and slowly infused into B6[CD45.2] host mice as described above. After 24 hours, the host mice were perfused with cool (4–8°C) physiological saline and the brain and systemic organs collected for analysis.
4.6. Immunohistochemistry
All tissues were immersion-fixed in buffered, 4% de-polymerized paraformaldehyde for 24 hours followed by cryoprotection in buffered 30% sucrose. The tissues were frozen, sectioned at 40μm thickness on a Leica CM3050 S cryostat, and mounted onto slides. Lavage samples of cells were dried onto slides and fixed with methanol. Selected specimens were immunostained with the following antibodies: 6E10 (1:5,000) (Covance), a mouse monoclonal antibody to human-sequence Aβ with an epitope at residues 3–8; rabbit polyclonal antibodies R361 and R398 (both at 1:15,000) (courtesy of Dr. Pankaj Mehta of the Institute for Basic Research on Developmental Disabilities, Staten Island, NY) to Aβ32–40 and Aβ33–42, respectively; and antibodies to CD45 (1:5000), CD45.1(1:1000) (BD Pharmingen), and laminin (1:1000) (Abcam ab11575). Specimens were washed in PBS, permeabilized using PBST (PBS and 0.2% Tween), and blocked for 1 h in 2% serum in PBST. Primary antibodies were added to PBST containing 2% serum and incubated with the cells or tissues overnight at 4°C while gently rocking. Secondary antibodies were added to PBST containing 2% serum and incubated with the specimens for 1.5 h at room temperature. In addition, some samples were counterstained with DAPI or hematoxylin. Cells and tissues were examined with a Leica DMLB microscope or an Olympus FV1000/TIRF inverted confocal microscope.
Highlights.
Exogenous macrophages can enter the brains of healthy host mice after taking up Aβ
Exogenous macrophages are found in the superficial neocortex and near blood vessels
Macrophages may act as vectors for the spread of proteopathic seeds
Acknowledgments
This work was supported by R36AG043646, R21AG040589, RR00165, T32 NS007480-14, and by the MetLife Foundation. We gratefully acknowledge Rajesh Nair (Emory University) and Mathias Jucker (University of Tübingen) for helpful discussions and advice.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguzzi A. Prions and the immune system: a journey through gut, spleen, and nerves. Adv Immunol. 2003;81:123–71. doi: 10.1016/s0065-2776(03)81004-0. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J. G Science, editor. Molecular Biology of the Cell. New York: 2002. The Adaptive Immune System. [Google Scholar]
- Angot E, Steiner JA, Lema Tome CM, Ekstrom P, Mattsson B, Bjorklund A, Brundin P. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS One. 2012;7:e39465. doi: 10.1371/journal.pone.0039465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JC, Dejoia C, Tucker T, Kincaid AE, Bessen RA. Extraneural prion neuroinvasion without lymphoreticular system infection. J Virol. 2005;79:11858–63. doi: 10.1128/JVI.79.18.11858-11863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–46. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA. Consequence of Abeta immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008;131:3299–310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015;129:221–37. doi: 10.1007/s00401-014-1373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Koliatsos VE, Guarnieri M, Pardo CA, Sisodia SS, Price DL. Rapid anterograde axonal transport of the cellular prion glycoprotein in the peripheral and central nervous systems. J Biol Chem. 1994;269:14711–4. [PubMed] [Google Scholar]
- Borrello MA, Phipps RP. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol Today. 1996;17:471–5. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–95. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- Buyukmihci N, Goehring-Harmon F, Marsh RF. Neural pathogenesis of experimental scrapie after intraocular inoculation of hamsters. Exp Neurol. 1983;81:396–406. doi: 10.1016/0014-4886(83)90271-6. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Grueninger F, Tolnay M. Intercellular transfer of tau aggregates and spreading of tau pathology: Implications for therapeutic strategies. Neuropharmacology. 2014;76(Pt A):9–15. doi: 10.1016/j.neuropharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- D’Alessandro A, Blasi B, D’Amici GM, Marrocco C, Zolla L. Red blood cell subpopulations in freshly drawn blood: application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 2013;11:75–87. doi: 10.2450/2012.0164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, Hu PP, Fedynyshyn J, Soto C. Aggregate-depleted brain fails to induce Abeta deposition in a mouse model of Alzheimer’s disease. PLoS One. 2014;9:e89014. doi: 10.1371/journal.pone.0089014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, Jucker M. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–31. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–2. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Fritschi SK, Hamaguchi T, Obermuller U, Fuger P, Skodras A, Schafer C, Odenthal J, Heikenwalder M, Staufenbiel M, Jucker M. Multiple factors contribute to the peripheral induction of cerebral beta-amyloidosis. J Neurosci. 2014;34:10264–73. doi: 10.1523/JNEUROSCI.1608-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CS, Frenzke M, Leonard VH, Welstead GG, Richardson CD, Cattaneo R. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150) J Virol. 2010;84:3033–42. doi: 10.1128/JVI.01559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–66. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- Fortier AH, Falk LA. Isolation of murine macrophages. Curr Protoc Immunol. 2001;Chapter 14(Unit 14):1. doi: 10.1002/0471142735.im1401s11. [DOI] [PubMed] [Google Scholar]
- Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84:225–33. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- Fritschi SK, Cintron A, Ye L, Mahler J, Buhler A, Baumann F, Neumann M, Nilsson KP, Hammarstrom P, Walker LC, Jucker M. Abeta seeds resist inactivation by formaldehyde. Acta Neuropathol. 2014;128:477–84. doi: 10.1007/s00401-014-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer’s disease: the blood-borne identity. J Neural Transm. 2010;117:961–70. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–73. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Devitt A. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology. 2004;113:1–14. doi: 10.1111/j.1365-2567.2004.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–31. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20:130–8. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbeck M, Nath S, Marcusson J. Neuron-to-neuron transmission of neurodegenerative pathology. Neuroscientist. 2013;19:560–6. doi: 10.1177/1073858413494270. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Eisele YS, Varvel NH, Lamb BT, Walker LC, Jucker M. The presence of Abeta seeds, and not age per se, is critical to the initiation of Abeta deposition in the brain. Acta Neuropathol. 2012;123:31–7. doi: 10.1007/s00401-011-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–30. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC, Belaygorod L, Cairns NJ, Holtzman DM, Diamond MI. Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci U S A. 2014;111:E4376–85. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–20. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT. Tau propagation, different tau phenotypes, and prion-like properties of tau. Neuron. 2014;82:1189–90. doi: 10.1016/j.neuron.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, Lee VM, Trojanowski JQ. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–8. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Michel BA, Meyerett C, Duffy A, Avery A, Dow S, Zabel MD. Monitoring immune cells trafficking fluorescent prion rods hours after intraperitoneal infection. J Vis Exp. 2010 doi: 10.3791/2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KS K, DLM, VJS, CMJC, CBI G-I, JRC, HMW Production and characterization of monoclonal antibodies reactive to synthetic cerebrovascular amyloid peptide. Neurosci Res Commun. 1988;2:121–130. [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–11. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol. 2009;183:1983–9. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–99. doi: 10.1097/00006123-199805000-00082. discussion 1099–100. [DOI] [PubMed] [Google Scholar]
- Lampert PW. Autoimmune and virus-induced demyelinating diseases. A review. Am J Pathol. 1978;91:176–208. [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J Neurosci. 2011;31:14488–95. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–6. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski PP, Yanagihara R, Gibbs CJ, Jr, Gajdusek DC. Spread of Creutzfeldt-Jakob disease virus along visual pathways after intraocular inoculation. Arch Virol. 1990;111:141–7. doi: 10.1007/BF01310512. [DOI] [PubMed] [Google Scholar]
- Liberski PP, Hainfellner JA, Sikorska B, Budka H. Prion protein (PrP) deposits in the tectum of experimental Gerstmann-Straussler-Scheinker disease following intraocular inoculation. Folia Neuropathol. 2012;50:85–8. [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–31. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol Commun. 2014;2:88. doi: 10.1186/s40478-014-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol. 2001;75:9320–7. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, Vigouret JM, Paganetti P, Walsh DM, Mathews PM, Ghiso J, Staufenbiel M, Walker LC, Jucker M. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–4. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–53. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. De novo induction of amyloid-beta deposition in vivo. Mol Psychiatry. 2012a;17:1347–53. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Diaz-Espinoza R, Camacho MV, Soto C. Protein misfolding cyclic amplification of infectious prions. Nat Protoc. 2012b;7:1397–409. doi: 10.1038/nprot.2012.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pertoft H. Fractionation of cells and subcellular particles with Percoll. J Biochem Biophys Methods. 2000;44:1–30. doi: 10.1016/s0165-022x(00)00066-x. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–61. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–35. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010 doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodig SJ, Shahsafaei A, Li B, Dorfman DM. The CD45 isoform B220 identifies select subsets of human B cells and B-cell lymphoproliferative disorders. Hum Pathol. 2005;36:51–7. doi: 10.1016/j.humpath.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81:343–50. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Fritz JJ, Dooyema J, Cintron AF, Hamaguchi T, Lah JJ, Levine H, 3rd, Jucker M, Walker LC. Exogenous seeding of cerebral beta-amyloid deposition in betaAPP-transgenic rats. J Neurochem. 2012;120:660–666. doi: 10.1111/j.1471-4159.2011.07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer’s disease. Neuroscience. 1987;23:389–98. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Scott JR, Davies D, Fraser H. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J Gen Virol. 1992;73(Pt 7):1637–44. doi: 10.1099/0022-1317-73-7-1637. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 2001;82:2327–34. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Stohr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, DeArmond SJ, Prusiner SB, Giles K. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc Natl Acad Sci U S A. 2012;109:11025–30. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr J, Condello C, Watts JC, Bloch L, Oehler A, Nick M, DeArmond SJ, Giles K, DeGrado WF, Prusiner SB. Distinct synthetic Abeta prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci U S A. 2014;111:10329–34. doi: 10.1073/pnas.1408968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Strazielle N, Gandy JR, Keefe TH, Zink BJ, Ghersi-Egea JF, Chodobski A. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J Cereb Blood Flow Metab. 2012;32:93–104. doi: 10.1038/jcbfm.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–9. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sadaike T, Inoshima Y, Ishiguro N. Characterization of PrP(Sc) transmission from immune cells to neuronal cells. Cell Immunol. 2012;279:145–50. doi: 10.1016/j.cellimm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–7. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–8. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- van Furth R, Sluiter W. Distribution of blood monocytes between a marginating and a circulating pool. J Exp Med. 1986;163:474–9. doi: 10.1084/jem.163.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. J Comp Pathol. 1999;121:55–63. doi: 10.1053/jcpa.1998.0300. [DOI] [PubMed] [Google Scholar]
- Walker LC, Levine H, 3rd, Mattson MP, Jucker M. Inducible proteopathies. Trends Neurosci. 2006;29:438–43. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, LeVine H., 3rd Corruption and spread of pathogenic proteins in neurodegenerative diseases. J Biol Chem. 2012;287:33109–15. doi: 10.1074/jbc.R112.399378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–10. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2011;108:2528–33. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Condello C, Stohr J, Oehler A, Lee J, DeArmond SJ, Lannfelt L, Ingelsson M, Giles K, Prusiner SB. Serial propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2014;111:10323–8. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Hamaguchi T, Fritschi SK, Eisele YS, Obermuller U, Jucker M, Walker LC. Progression of seed-induced Abeta deposition within the limbic connectome. Brain Pathol. 2015 doi: 10.1111/bpa.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood-brain barrier. J Neurochem. 2001;76:1597–600. doi: 10.1046/j.1471-4159.2001.00222.x. [DOI] [PubMed] [Google Scholar]