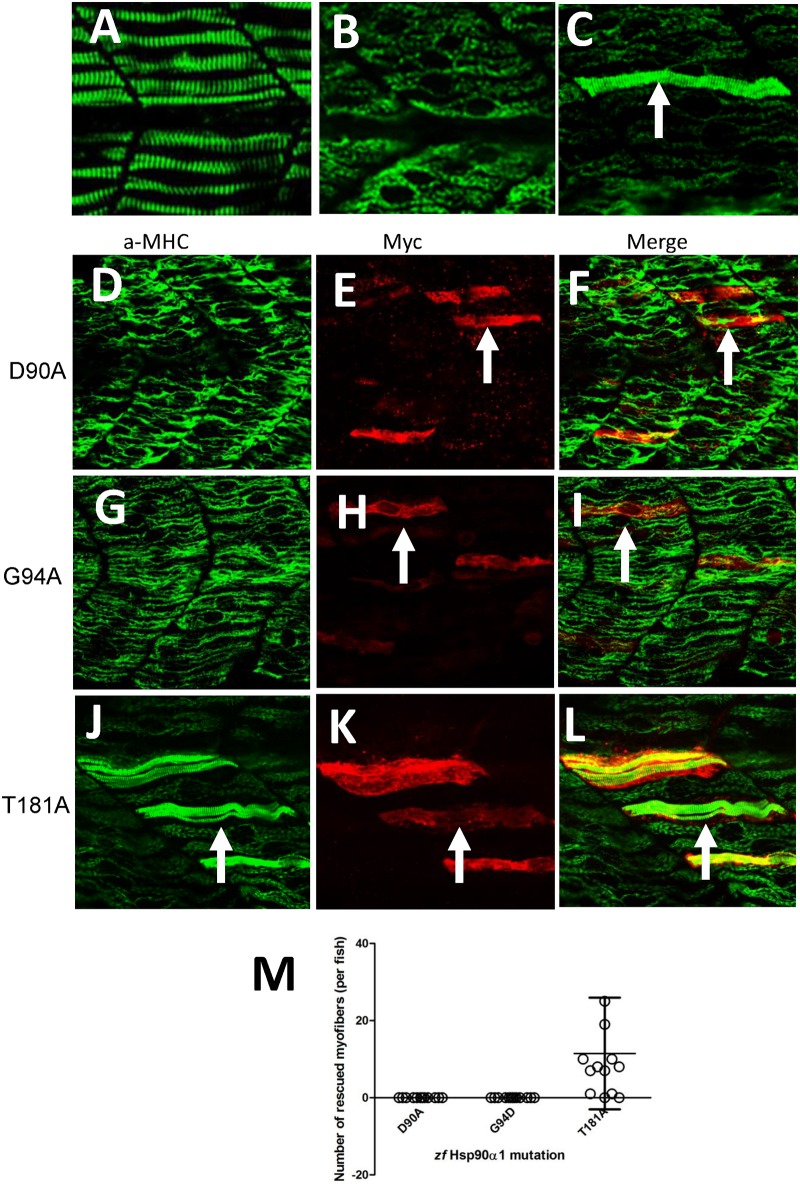
Fig 2. Mutating D90, G94 but not T181 in the N-terminal ATP binding domain disrupts Hsp90α1 function in myosin thick filament organization.
DNA construct expressing the myc-tagged wild type Hsp90α1, D90A, G94D or T181A mutant was co-injected with Hsp90α1 ATG-MO into fertilized eggs of zebrafish. The injected embryos were analyzed by double staining with anti-myc (9E10) and anti-MHC (F59) antibodies at 28 hpf. A-C. Anti-MHC antibody staining shows the thick filament organization in skeletal muscles of control (A), Hsp90α1 knockdown (B), or DNA and ATG-MO co-injected (C) embryos. D-F. Myosin thick filament organization and myc-tagged D90A expression in skeletal slow muscles of a zebrafish embryo co-injected with Hsp90α1 ATG-MO and D90A construct. G-I. Myosin thick filament organization and myc-tagged G94D expression in skeletal slow muscles of a zebrafish embryo co-injected with Hsp90α1 ATG-MO and G94D construct. J-L. Myosin thick filament organization and myc-tagged T181A expression in skeletal slow muscles of a zebrafish embryo co-injected with Hsp90α1 ATG-MO and T181A construct. M. Plot showing the number of rescued myofibers in 12–13 individual zebrafish embryos injected with D90A, G94D or T184A mutant. Scale bar = 20 μm.