Scheme 4.
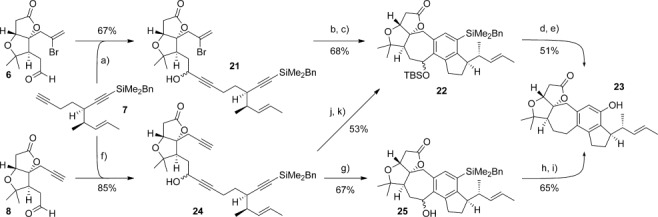
Reagents and conditions: a) nBuLi, 7, −78 °C; then add 6, −78 °C→−10 °C, 2 h, 67 %; b) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C→RT, 4 h 75 %; c) [Pd(PPh3)4] (10 mol %), Et3N, MeCN, 80 °C, 18 h, 91 %; d) TBAF, THF, RT, 30 min; then H2O2, KHCO3, MeOH, RT, 12 h; e) Et3SiH, ZnCl2, CH2Cl2, RT, 3 h; then TBAF, THF, RT, 20 min, 51 % (2 steps); f) nBuLi, 7, −78 °C; then add 8, −78 °C→−10 °C, 4 h, 85 %; g) [CpCo(CO)2] (20 mol %), PPh3 (40 mol %), PhCl, MW (300 W), 150 °C, 25 min, 67 %; h) TBAF, THF, RT, 30 min; then H2O2, KHCO3, MeOH, RT, 12 h, 84 %; i) Et3SiH, ZnCl2, CH2Cl2, RT, 3 h, 77 %; j) TBSCl, imid., DMAP, CH2Cl2, RT, 6 h, 98 %; k) [CpCo(CO)2] (20 mol %), PPh3 (40 mol %), PhCl, MW (300 W), 150 °C, 25 min, 54 %. Cp=cyclopentadienyl, MW=microwave irradiation, OTf=trifluoromethanesulfonate, TBAF=tetra-n-butylammonium fluoride.
