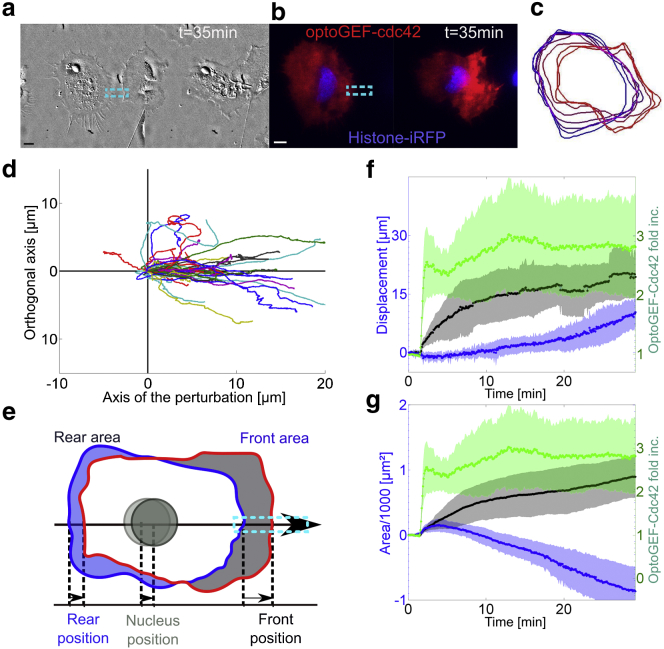
Figure 7.
Quantitative measurement of HeLa cell movement in response to optoGEF-Cdc42 gradient. (a and b) Local activation of Cdc42 in a HeLa cell expressing OptoGEF-Cdc42 and H2B-iRFP. DIC images (a) and fluorescent images (b) showing mCherry (red) and iRFP (blue) at two time points, before (left) and 28 min after activation (right). (Dashed-blue rectangles) Area of blue illumination. (c) Outline of the cell for increasing time points (from blue to red) over 30 min. The outer border of the cell was segmented from TIRF mCherry images every 4 min. (d) Quantification of the displacement of the cell barycenter for 30 min moves along and perpendicularly to the main axis of the cell for N = 36 cells. The main axis is defined by a line passing through the cell barycenter at t = 0 min and the position of the recruitment. (e–g) Quantification of cell movement induced by local Cdc42 activation for N = 5 cells. (e) Scheme of the different elements being quantified: cell front, rear, and nucleus displacement along the migration axis and the evolution of the areas of the front and the rear. (f and g) Quantification of OptoGEF-Cdc42 in the photoactivated region (green) and of the barycenter displacement (f) and area (g) of the front (black) and the rear (blue) of the cell. The photoactivation was done with six pulses of 50 ms every 5 s, followed by pulses of 50 ms every 25 s. (Shaded areas) Mean ± SD (dots). To see this figure in color, go online.