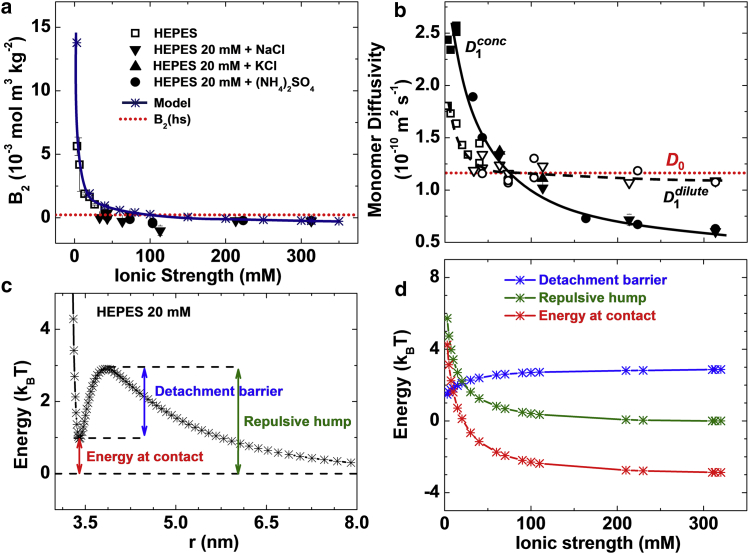
Figure 3.
Characterization of the intermolecular interactions in solution at pH = 7.8. (a) The dependence of the second osmotic virial coefficient B2 on the ionic strength I, varied through the concentration of HEPES buffer or by adding KCl, NaCl, or (NH4)2SO4. The values of B2 computed using the model represented in Fig. S3 are also shown. B2(hs) = 4 VMNAMw−2 = 2.35 × 10−4 mol m3 kg−2 for hard spheres (VM = 2.0 × 10−26 m3, molecular volume; NA, Avogadro’s number; Mw = 14.5 kg mol−1, lysozyme molecular weight) is shown for comparison. (b) The dependence of monomer diffusivity in dilute (D1dilute, measured at 9 mg mL−1, open symbols) and concentrated (D1conc, measured at 100 mg mL−1, solid symbols) solutions on the ionic strength, varied by the addition of the same electrolytes as in (a). The Stokes-Einstein diffusivity (or self-diffusivity) D0 = 1.20 × 10−10 m2 s−1 of a sphere of radius 1.7 nm in a solution with viscosity 1.06 mPa s is shown. Solid and dashed lines are guides for the eye. (c) Potential of mean force (PMF) between a pair of molecules as a function of the distance between their centers of mass calculated using a numerical model illustrated in Fig. S3 at ionic strength I = 13.3 mM. (d) The dependences of the energy at contact, repulsive hump, and detachment barrier, defined in (c) on the ionic strength. To see this figure in color, go online.