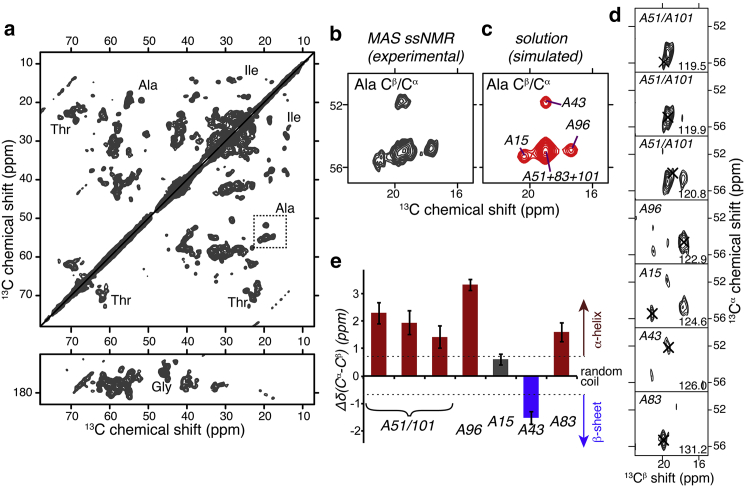
Figure 4.
2D and 3D MAS NMR. (a) 2D 13C-13C spectrum on 1.7 mg oxidized 13C,15N-cyt-c bound to DOPC/TOCL LUVs, obtained with 10 ms DARR mixing. Selected residue types are indicated and the dashed box marks Ala Cα-Cβ crosspeaks. The spectrum was processed with fourfold zero-filling after 15 Hz Lorentzian line sharpening and 40 or 50 Hz Gaussian line broadening in the direct and indirect dimensions, respectively. (b) Enlargement of the Ala Cβ-Cα crosspeaks; (c) Analogous (simulated) peaks from solution NMR data (46) on the soluble protein (see text for details). (d) 2D slices from a 3D NCACX spectrum reveal distinct Ala signals (black X), at 15N shifts shown at bottom right. Tentative assignments based on correspondence to solution NMR shifts are indicated. (e) MAS NMR secondary chemical shifts Δδ(Cα-Cβ) deviate from random coil values (at zero) and indicate mostly α-helical structure for the Ala, in line with the native structure (Fig. 1a). Dashed lines at ±0.7 ppm indicate the typical boundaries to the random coil regime. Experiments performed at 233 K, 8.33 kHz MAS, and 600 MHz (for 1H). To see this figure in color, go online.