Figure 1. Selective inhibition of ERK-mediated phosphorylation of substrates by compounds.
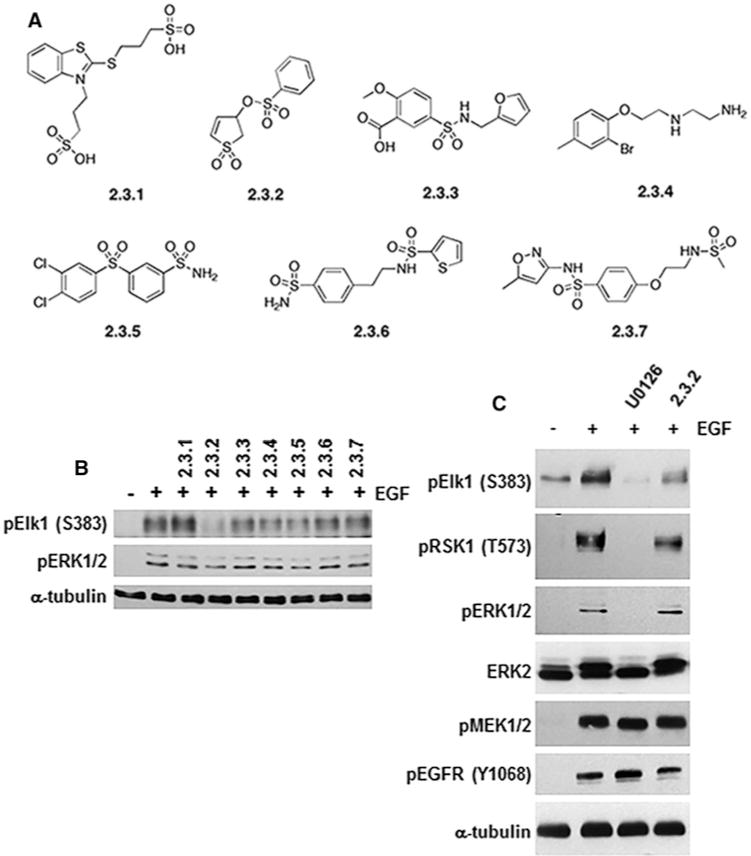
(A) Structures of the seven diverse compounds identified by CADD and initially tested. (B) HeLa cells were pre-incubated for 30 min with indicated compounds (100 μM) followed by treatment with EGF (25 ng/ml) for 10 min to activate ERK1/2 signalling. Lysates were immunoblotted for phosphorylated Elk-1 (pElk-1 Ser383) or active ERK1/2 (pERK1/2). (C) Following treatment as in (B), immunoblot analysis of lysates from cells treated with 2.3.2 suggested selective phosphorylation inhibition of Elk-1 as compared with RSK1 (pElk-1 Ser383 and pRSK1 Thr573). Phosphorylation of ERK1/2 and, MEK1/2 (pERK1/2 and pMEK1/2) or Tyr1068 autophosphorylation of EGFR (pEGFR Tyr1068) was not affected by 2.3.2. The MEK1/2 inhibitor U0126 (10 μM) was used to inhibit all ERK1/2 signalling, and α-tubulin expression was used as a protein loading control.
