Abstract
Objective Examine treatment adherence rates in pediatric eosinophilic gastrointestinal disorders (EGID). Methods Participants were children aged 2.5–18 years with eosinophilic esophagitis or eosinophilic gastroenteritis (EGE) and their caregivers. A multimethod, multi-informant assessment including parent report and electronic monitoring was utilized, with a 90% cut point for nonadherence. Results Medication nonadherence prevalence was 30%. Adherence frequency was 91% ± 14% (0–100%) per parent report and 100% ± 69% (0–194%) per electronic monitors. Tube-feeding adherence was 99% ± 3%. Food allergen exposures were less than 1 per 2 weeks, with 33% nonadherence prevalence. Patients with EGE and toddlers with both conditions demonstrated poorer medication adherence (p’s < .05). Caregivers reported higher number of missed medication doses than food exposures (p < .05). Conclusions The prevalence and range of nonadherence demonstrates that subsets of these patients are nonadherent. Adherence to treatment in EGID is complex and multifaceted, with nonadherence varying across treatments.
Keywords: adherence, compliance, eosinophil, gastrointestinal
Introduction
Pediatric eosinophilic gastrointestinal disorders (EGID) are a group of chronic gastrointestinal tract inflammatory conditions that often require long-term medication and severe dietary restrictions of allergic foods. An endoscopic procedure is needed to take biopsies of the mucosa in the GI tract to establish the specific diagnosis. Two of the more common EGID conditions are eosinophilic esophagitis (EoE) and eosinophilic gastroenteritis (EGE). While EoE is characterized by elevated levels of eosinophils [i.e., 15 eosinophils/hpf (peak value) is considered a minimum threshold for a diagnosis of EoE] (Liacouras et al., 2011) and inflammation in the esophagus, EGE inflammation and eosinophil concentration is located primarily in the stomach and/or small intestine. In general, a diagnosis of EGE is based on above normal levels (DeBrosse, Case, Putnam, Collins, & Rothenberg, 2006) of eosinophils and their location in the GI tract. Patients with EoE experience symptoms including dysphagia, pain, food impaction, gastroesophageal reflux disease (GERD), and vomiting; patients with EGE may experience symptoms of pain, vomiting, and diarrhea. While there is no published practice guidelines for treatment of these disorders, an updated consensus recommendations for treatment has recently been published (Liacouras, et al., 2011). Therapies for these conditions include, but are not limited to, oral medication in pill form, swallowed steroid therapies (e.g., swallowed fluticasone using a metered dose inhaler designed for asthma treatment) to topically coat the affected GI mucosal surfaces to reduce inflammation and dietary food antigen elimination diets to address underlying food allergies as the principal etiology for these conditions. As the prevalence of pediatric EGID continues to increase (Liacouras, et al., 2011), management of these conditions represents an important concern for pediatric healthcare professionals. Treatment regimens for EGID are often complex, demanding, and require organization, cooperation, and planning by both patients and their parents. These treatments are further complicated by potential side effects of medications, ease with which prohibited foods can be obtained, patient versus parent responsibility for treatments, and the financial and social costs. Yet, to date there have been no published studies examining adherence in children with EGID.
Nonadherence to prescribed treatment regimens is a common issue across pediatric chronic illness populations, with nonadherence prevalence estimates of 50% in children (Rapoff, 2010) and 75% (Logan, Zelikovsky, Labay, & Spergel, 2003) to 88% (Hommel, Davis, & Baldassano, 2009) in adolescents. Although there are no known studies of nonadherence in EGID, data from inflammatory bowel disease (IBD) and asthma, which are managed via oral medication, and celiac disease, which is managed via dietary restriction therapies, offer insight into the potential adherence problems that patients with EGID may experience. In pediatric asthma, nonadherence in children is common. Walders and colleagues (Walders, Kopel, Koinis-Mitchell, & McQuaid, 2005) reported a 54% rate of nonadherence to daily medication. Nonadherence to inhaled corticosteroids in children and adolescents has been documented at 51% (McQuaid, Walders, Kopel, Fritz, & Klinnert, 2005) and 48% (McQuaid, Kopel, Klein, & Fritz, 2003). In addition, Bauman and colleagues (Bauman et al., 2002) reported that 45% of young children with asthma either did not have a prescription filled or was given more than or less than what was prescribed. Nonadherence rates in pediatric IBD and celiac disease range from 16% (Ooi, Bohane, Lee, Naidoo, & Day, 2007) to 62% (Mackner & Crandall, 2005) in IBD for medication nonadherence and from 5% (Rashid et al., 2005) to 70% (Westman, Ambler, Royle, Peat, & Chan, 1999) in celiac disease (Hommel, Mackner, Denson, & Crandall, 2008) for gluten-free diet nonadherence. Using both patient- and parent-report interview assessments of medication nonadherence in adolescents with IBD, Mackner and Crandall (2005) found nonadherence (defined as <100% of medication consumed) rates of 57–62% according to adolescent and parent reports, respectively. Our research, using both subjective (i.e., self-report) and objective (i.e., pill counts) assessment methods, has documented nonadherence prevalence rates of 64–88% depending on medication type and nonadherence frequency rates of 38–49% in adolescents with IBD (Hommel et al., 2009). Thus, there is empirical evidence that nonadherence is prevalent and frequent in two gastrointestinal conditions that involve treatments similar to those that patients with EGID are prescribed. Documenting rates of nonadherence in patients with EGID will address a potentially significant aspect of clinical care that may have substantial impact on health outcomes (e.g., uncontrolled GI tract inflammation, esophageal food impaction, development of an esophageal stricture, poor growth, pain, worsening symptoms, etc.).
Examination of disease management issues, particularly nonadherence, therefore, represents a significant need in the pediatric EGID population. Accordingly, the present study was designed to determine the prevalence and frequency of treatment nonadherence, including medication, tube feeding, and dietary restrictions, in a cohort of children with EGID using multi-informant parent-report (i.e., maternal and paternal reporting) assessment of adherence (as well as objective electronic monitoring in a subsample). Using this approach, we conceptualized nonadherence on a continuum by examining frequencies, but also acknowledged, via examination of prevalence, the clinical importance of distinguishing adherent versus nonadherent patients. We hypothesized that a substantial proportion of patients would demonstrate nonadherence and that nonadherent behavior would be evident across treatment types (i.e., oral medication, tube feedings, and dietary restriction therapies).
Methods
Participants
This study was performed with the approval of the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board. Patients with EGID were recruited from local and referral populations at CCHMC and its Cincinnati Center for Eosinophilic Disorders (CCED). Inclusion criteria were (a) patients age 2.5–18 years and (b) primary diagnosis of EGID, including EoE and EGE. Exclusion criteria were (a) diagnosis of severe developmental delay as evidenced by chart review (due to limited comprehension of questionnaires) and (b) diagnosis of a chronic condition other than EGID. Eligible participants that were recruited consisted of (a) new patients seen in the CCED, (b) existing patients followed in the outpatient gastroenterology clinic, or (c) existing patients attending a national educational conference for patients diagnosed with EGID and their families. These patients’ medical records were reviewed to ensure that they met inclusion criteria. Of the 116 families contacted, nine declined participation (five questionnaires took too much time, two not interested in research, and two family overwhelmed with clinic visit), one withdrew (did not have enough time to complete questionnaires), one was diagnosed with reflux instead of EGID, one was excluded from analyses due to procedural error, and eight did not return complete data. Therefore, the final sample included 96 patients with EGID and their caregivers.
Study Design and Procedures
We conducted a cross-sectional study of EGID patients and their families who were identified during clinic or endoscopy appointments with the cohort ranging from initial diagnosis to well-established patients. Participants provided informed consent and caregiver and patient age-specific assessments were conducted during one visit. Assessments were conducted according to appointment type (i.e., clinic appointment, outpatient surgery, or conference meeting) and time available for completion to accommodate patient and family schedules. If the family did not have sufficient time to complete all assessments in person, they were provided with a prepaid, addressed envelope to complete forms at home and return by mail. Study staff conducted follow-up phone calls to collect any outstanding assessments. Patients who were prescribed swallowed corticosteroid therapy via a metered-dose inhaler (MDI) were given MediTrack Doser electronic monitor devices to monitor three months of medication adherence. Electronic monitors were mailed to study staff after completion of each 30-day period, and data were transferred to a database for analysis.
Measures
Caregivers completed a demographic form providing data on caregiver ages, education, marital status, employment status, and household income.
EGID Treatment Adherence Questionnaire
The Treatment Adherence Questionnaire (TAQ) was developed specifically for the study to assess the unique adherence factors involved in treatment of EGID. It is a 12-item parent report questionnaire that measures patient adherence to both medication and dietary restriction regimens. Factors assessed include medication adherence, timing of missed medications, adherence to dietary restriction recommendations (defined as refraining from consuming unwanted food allergen exposures such as eating a food that unknowingly contained an allergenic ingredient), tube-feeding adherence, and responsibility for completing treatments. Single-item measurement is used to assess each of these factors. Example questions include:
Children and adolescents often have difficulty taking medications and doing tube feedings. They may forget, have activities that conflict with the treatment, or just decide not to take a dose of medication or do a tube-feeding treatment. There may be other reasons too. All of these reasons are completely understandable.
Please tell us the number of medication doses your child/adolescent has missed in the past two weeks: _____.
Please tell us the number of tube feeding treatments your child/adolescent has missed in the past two weeks: _____.
MediTrack Dosers
MediTrack Doser electronic devices were attached to the top of a patient’s MDI and recorded the number of compressions exhausted from the MDI. The Doser device consists of an LCD screen that attaches to the top of the MDI and displays the number of compressions daily, as well as the number of inhalations remaining in the canister. Dosers record a maximum of 30-days of data. Patients were provided three Dosers to track three months of adherence. Thirteen patients were prescribed swallowed corticosteroid therapy via a MDI at the time of consent. Data were available for 12 patients as one did not use the electronic monitor as requested. Similar to other electronic monitors, Dosers have the potential to fail to record inhalations or may record additional compressions of the MDI that were not inhaled.
Data Analyses
Raw data were entered into a secure database and data quality analysis was performed. All data analyses were conducted in PASW 18.0. Parent-reported adherence data were available for all prescribed medications; Doser electronic data were also available for a subsample of patients prescribed swallowed fluticasone. Descriptive statistics were calculated for demographic information, parent-report adherence data, and electronic monitor adherence data. Data were compared using independent samples t-tests and paired samples t-tests. A multivariate analysis of variance was also conducted to examine differences in adherence based on age, categorized as toddler (ages 2–4 years; N = 26), young child (ages 5–7 years; N = 25), child (ages 8–12 years; N = 28), and adolescent (ages 13–18 years; N = 17). These categories are consistent with prior studies in pediatric populations (Varni, Seid, & Kurtin, 2001). All tests were considered significant at the p < .05 level. Dosers record for 30 days then automatically overwrite (i.e., return to Day 1 to re-record) days until data are recorded. To control for this overwriting, Doser data were truncated, with the first 5 days of each month deleted. This provided a more conservative approach to data interpretation. The cut point for nonadherence was set at 90% across adherence behaviors assessed (e.g., medication, diet, etc.). This value is slightly higher than commonly seen in adherence research. Although an 80% cut point is often used, it is strictly arbitrary and not disease- or treatment specific or tied to clinical outcome. Additionally, 90% was used in this study because of the potentially significant consequences of nonadherence (e.g., uncontrolled GI tract inflammation, esophageal food impaction, anaphylaxis, development of an esophageal stricture, poor growth, and pain) in EGID compared to other chronic disease groups which have less immediate and/or severe outcomes associated with nonadherence.
Results
Patient Demographics
Family demographic and patient disease parameters for this study included patient age, gender, ethnicity, diagnosis, primary and secondary caregiver age, relation to patient, marital status, employment status, education level, and annual family income. Descriptive data for these variables are shown in Table I.
Table I.
Demographic and Disease-Related Descriptive Data
| N | 96 |
|---|---|
| Patient age (years) | 8.31 ± 4.33; range = 2.05–18.37 |
| Patient gender (% male) | 77.1 |
| Ethnicity (%) | |
| Caucasian | 84.4 |
| African American | 2.1 |
| Hispanic | 2.1 |
| Asian | 2.1 |
| Biracial | 8.3 |
| Other | 1.0 |
| Primary caregiver relation to patient (%) | |
| Biological Mothers | 95.8 |
| Biological Father | 4.2 |
| Primary caregiver age | 39.70 ± 5.82; range = 27.36–55.23 |
| Primary caregiver marital status (percent married) | 89.6 |
| Primary caregiver education level (percent with at least some college education) | 71.9 |
| Primary caregiver employment status (%) | |
| Employed part time | 27.1 |
| Employed full time | 32.3 |
| Secondary caregiver relation to patient (%) | |
| Biological father | 82.3 |
| Biological mother | 4.2 |
| Stepfather | 3.1 |
| Grandmother | 2.1 |
| Secondary caregiver age | 42.11 ± 6.84; range = 28.31–69.25 |
| Secondary caregiver marital status (percent married) | 95.5 |
| Secondary caregiver education level (percent with at least some college education) | 66.7 |
| Secondary caregiver employment status (%) | |
| Employed part time | 1.1 |
| Employed full time | 93.2 |
| EGID Diagnosis (%) | |
| Eosinophilic esophagitis | 85.4 |
| Eosinophilic gastroenteritis | 14.6 |
| Annual family income (%) | |
| $0–$25,000 | 2.1 |
| $25,001–$50,000 | 9.4 |
| $50,001–$75,000 | 12.5 |
| $75,001–$100,000 | 19.8 |
| $100,001–$125,000 | 18.8 (median) |
| $125,001–$150,000 | 11.5 |
| $150,001–$175,000 | 4.2 |
| $175,001–$200,000 | 2.1 |
| Over $200,000 | 16.7 |
Medication Adherence by Parental Report and Electronic Monitor Data
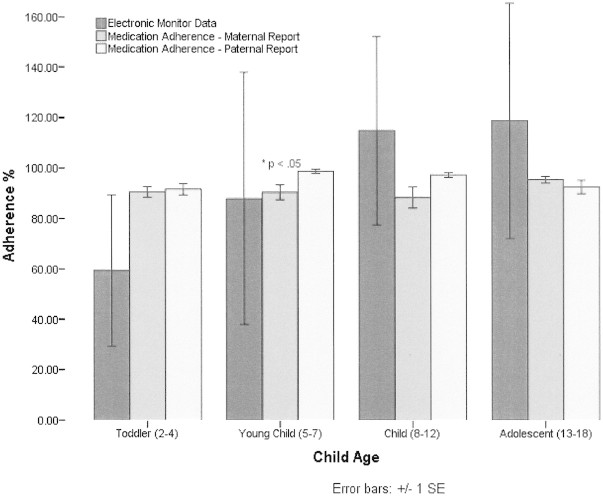
Mean medication adherence frequency per maternal report (N = 85) was 91% ± 14%; paternal report (N = 42) was 95% ± 6%. Thirty percent of the sample was nonadherent per maternal report and 15% per paternal report. Importantly, the range of medication nonadherence was 0–100% for maternal report and 79–100% for paternal report, demonstrating substantial variability in medication adherence in this sample. A paired samples t-test was conducted to examine differential ratings by parents and revealed nonsignificant differences between maternal and paternal ratings (p > .05). Independent samples t-test revealed a significant difference between EoE (N = 82) and EGE (N = 14) patients on maternal report of medication adherence (t = −2.28, p < .05), with EGE patients demonstrating poorer adherence (Figure 1). Additionally, a significant difference was observed between age groups based on paternal report of medication adherence (F = 3.64, p < .05), with toddlers with both conditions demonstrating poorer adherence than young children (Figure 2).
Figure 1.
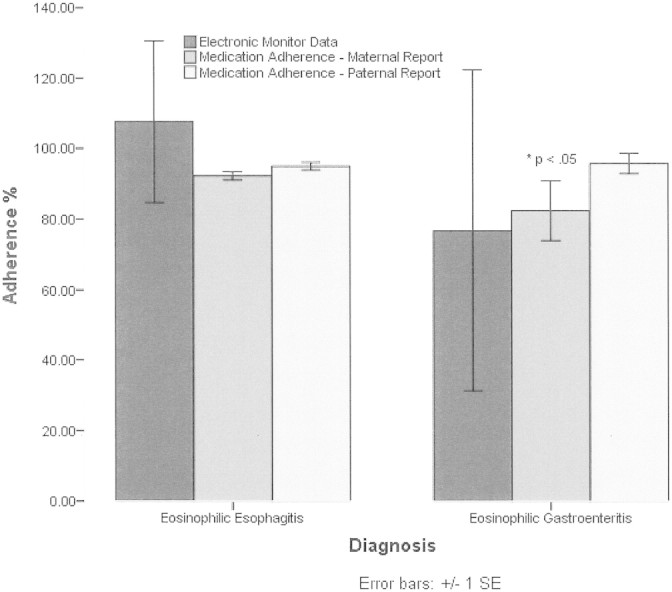
Medication adherence frequency for patients by diagnosis. Asterisks indicate EoE and EGE patients significantly different at p < .05 for medication adherence–maternal ratings.
Figure 2.
Medication adherence frequency for patients by age. Asterisks indicate toddlers and young children significantly different at p < .05 for medication adherence–paternal ratings.
Analysis of the Doser electronic monitor data (N = 12) revealed 100% ± 69% adherence; however, this also revealed a substantial range of 0–194%, with approximately one-half of those patients overdosing. Thus, nonadherence was bidirectional with swallowed fluticasone resulting in both underdosing and overdosing (Figure 3).
Figure 3.
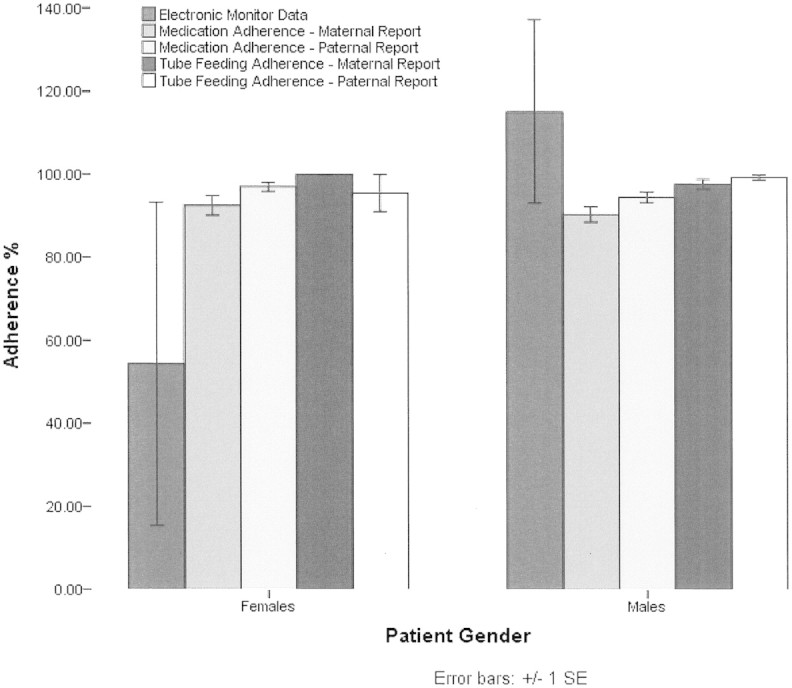
Medication and tube-feeding adherence frequency for patients by gender.
Tube Feeding Adherence by Parental Report
Twelve patients were prescribed tube-feeding treatment. Adherence to this treatment was 99% ± 3% per maternal report and 98% ± 4% per paternal report. Thus, maternal and paternal ratings were congruent for tube-feeding adherence. The range of adherence was 91–100%, with 75% of mothers and 67% of fathers reporting 100% adherence (Figure 3). Differential ratings by parents were examined using a paired samples t-test, which revealed nonsignificant differences between maternal (N = 12) and paternal (N = 5) ratings (p > .05).
Dietary Adherence by Parental Report
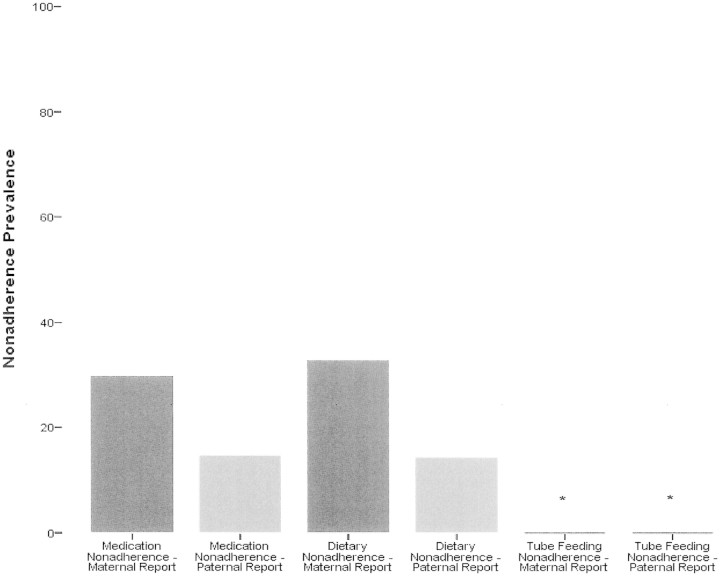
Food allergen exposures were calculated as whole numbers because there was no denominator. That is, because dietary restriction represents an absence of a behavior, there is no prescribed number of behavioral events by which to divide. Mean maternal (N = 71) and paternal (N = 43) reports of unwanted food allergen exposures portrayed similar rates with 0.87 and 0.40 exposures over the previous two weeks, respectively. Thirty-three percent and 14% of the patients reported at least one exposure during the previous 2 weeks per maternal and paternal report, respectively (Figure 4). There was also general consistency in report of accidental food allergen exposures, with mothers reporting 0.26 and fathers reporting 0.37 accidental food allergen exposures over the previous two weeks. A paired samples t-test revealed nonsignificant differences between maternal and paternal ratings (p > .05). Number of food allergen exposures did not differ significantly based on diagnosis of anaphylaxis (p > .05).
Figure 4.
Nonadherence prevalence by treatment type. Asterisks indicate tube-feeding nonadherence prevalence = 0.
The number of missed medication doses was also compared to food allergen exposures to determine if differences in adherence behavior existed based on type of treatment. Both maternal and paternal reports of missed medication doses were compared to food allergen exposures via paired samples t-tests. Missed medication doses per maternal report (N = 68) (2.38 ± 3.25) were significantly higher than mean food allergen exposures (0.88 ± 1.93), t = −3.47, p < .01. Similarly, per paternal report (N = 37), missed medication doses (1.24 ± 1.52) were significantly higher than mean food allergen exposures (0.46 ± 1.19), t = 2.47, p < .05.
Medication Organization and Allocation of Treatment Responsibility
Medications were most frequently kept in the household kitchen cupboard or on the kitchen counter, per maternal (69%) and paternal (57%) report. Only 17% of mothers and 18% of fathers reported using a pill box to organize their child’s medications, although this may reflect prescription rates of medication in pill form for treatment of EGID. No maternal or paternal reporters indicated keeping medications in parent’s bedroom. Findings regarding allocation of treatment responsibility are detailed in Table II. There was good agreement between mothers and fathers regarding mothers’ level of treatment responsibility across treatment-related tasks; however, fathers rated their level of responsibility slightly higher than mothers rated fathers’ responsibility.
Table II.
Allocation of Treatment Responsibility per Parent Report
| Who is in charge of making sure your child’s medications have been taken? | Maternal report n = 90 (%) | Paternal report n = 51 (%) |
|---|---|---|
| Mother | 92.2 | 96.1 |
| Father | 60.0 | 82.4 |
| Child/Adolescent | 18.9 | 13.7 |
| Grandmother | 6.7 | 5.9 |
| Grandfather | 2.2 | 3.9 |
| Older sibling of patient | – | 2.0 |
| Not applicable | 2.2 | – |
| Who is in charge of making sure your child’s dietary recommendations are followed? | Maternal report n = 90 (%) | Paternal report n = 51 (%) |
|---|---|---|
| Mother | 93.3 | 94.0 |
| Father | 68.9 | 78.0 |
| Child/Adolescent | 14.4 | 12.0 |
| Grandmother | 10.0 | 6.0 |
| Grandfather | 6.7 | 2.0 |
| Not applicable | 5.6 | 4.0 |
| Who is in charge of making sure your child’s tube feeding treatments have been completed? | Maternal report n = 12 (%) | Paternal report n = 12 (%) |
|---|---|---|
| Mother | 100 | 100 |
| Father | 91.7 | 100 |
| Child/Adolescent | 33.3 | – |
| Grandmother | 16.7 | – |
| Older sibling | 8.3 | 20.0 |
| Not applicable | – | – |
| Who is in charge of getting your child’s medicine? | Maternal report n = 90 (%) | Paternal report n = 51 (%) |
|---|---|---|
| Mother | 97.8 | 94.1 |
| Father | 45.6 | 68.6 |
| Child/Adolescent | 3.3 | 5.9 |
| Grandmother | 4.4 | 2.0 |
| Not applicable | 2.2 | – |
Discussion
This study is the first to examine nonadherence in children diagnosed with an EGID. The findings indicate that there is a 30% nonadherence prevalence rate for medication therapy based on parental report, with a nonadherence frequency range of 0–100% and a range of 0–194% based on electronic monitor data. In contrast, tube-feeding adherence was remarkably high (99%) with little variability. Dietary adherence data revealed that on average, patients were exposed to less than one food allergen that they were supposed to be avoiding in the previous two weeks; however, there was a 33% prevalence of dietary nonadherence (i.e., primary caregivers reporting at least one exposure in previous 2 weeks). Significant differences in medication adherence were found for patient diagnosis, with EGE patients demonstrating poorer adherence. Toddlers demonstrated poorer medication adherence than young children. Missed medication doses were significantly higher than number of food allergen exposures per both maternal and paternal report.
A substantial proportion of the patients in this sample demonstrated nonadherence to medication and dietary recommendations. This, combined with the observed range of medication nonadherence, demonstrates that subsets of these patients have particular difficulty adhering to treatment regimens. Moreover, there was objective evidence of both underdosing and overdosing with swallowed corticosteroid therapy, indicating bidirectional nonadherence. While adherence to dietary recommendations was better than medication regimens, patients were still being exposed to food allergens and only a small proportion of those exposures were accidental. Thus, dietary adherence may also be problematic; however, objective assessment of dietary patterns is warranted to further articulate issues concerning adherence to this treatment approach. In contrast, EGID patients are able to adhere to tube-feeding treatments well, with very little variability. EGE patients may have more difficulty adhering to medication than EoE patients, which may suggest a difference in perception of symptom relief between patient diagnostic groups. Medication adherence in toddlers may be more challenging than other age groups, which might represent behavioral challenges to getting toddlers to take medications. Also the comparison of missed medication doses and dietary adherence suggests that medication adherence may be more difficult for families. The majority of participants reported effective organizational strategies for managing their treatments and mothers were uniformly rated as being primarily responsible for making sure medications were taken and refilled, dietary recommendations were followed, and tube feedings were completed. These findings regarding organizational factors and treatment responsibility have important clinical implications. For example, attention to improving organizational strategies may not result in considerable improvement in adherence. However, given that disease management in this population is primarily done by mothers, focusing on transition of responsibility at a developmentally appropriate time in the patients’ lives should help with long-term management into adulthood. In addition, the findings of this study suggest that clinicians should focus most of their self-management support on medication and dietary adherence compared to tube-feeding adherence as the latter does not appear to be problematic based on these initial results. Assessing difficulties in adhering to medication and diet regularly, assessing barriers, and developing action plans for overcoming these barriers may be particularly helpful. Further, providing families with toddler-age patients with specific behavior management strategies (e.g., reward contingencies, differential attention, and positive reinforcement for desired behaviors) that may impact medication adherence would likely be quite beneficial. Collectively, the findings of this initial investigation suggest that adherence to treatment in EGID is complex and multifaceted, with patients level of nonadherence varying across treatments.
The examination of multiple types of adherence, which cover the most common treatments for EGID, allowed identification of nonadherence frequency across a range of treatments. Sampling from both local and referral populations as well as conference attendees provided a potentially broader pool from which patients were sampled. Inclusion of objective electronic monitor assessment for medication adherence provided the opportunity to supplement parent-report data and to observe bidirectional nonadherence that would not otherwise have been revealed. Notably, pediatric behavioral science has historically neglected examination of paternal data and there is an increasing emphasis on the inclusion of fathers in observational and treatment research (Phares, Lopez, Fields, Kamboukos, & Duhig, 2005). Our use of both maternal and paternal informants for adherence data allowed for evaluation of discrepancies in reporting of adherence rates. However, these findings must also be interpreted within the context of a few limitations, including the use of parent-report adherence assessment data. While subjective assessment is particularly common in exploratory investigations of adherence in novel populations like this, self- and parent-report adherence data generally represents overestimations of adherence (Rapoff, 2010). Thus, our observed parent-report adherence rates may be generous estimates of adherence and should be substantiated via future research utilizing objective methods. In addition, given the small N and consequent cell sizes for tube feeding and electronic monitor adherence as well as the small number of EGE patients in the study, generalization of these findings is not yet warranted until replication with a larger sample can be conducted. This sample also comprised primarily male patients who were Caucasian and came from middle-to-upper socioeconomic backgrounds, with parents who were mostly married, educated, and employed. Future research on adherence in this population should focus on diversifying the samples to the extent possible. Finally, the cross-sectional nature of the study precluded examination of trajectories of adherence over time. This will be necessary to determine behavioral patterns that require intervention to improve disease management skills.
Future research should focus on longitudinal assessment of adherence using a multimethod approach comprising objective and subjective (e.g., dietary interview data, 24-hr random recall of diet, patient self-report, etc.) methods. This will enable predictive modeling via examination of trajectories of adherence and related clinical outcomes as well as identification of optimal timing for intervention (e.g., when adherence is likely to decrease). This research should also focus on the relationship between adherence and disease outcomes such as symptom severity, healthcare utilization, etc., which will aid in identifying the most accurate cut point for identification of nonadherent patients for whom intervention will be most needed. Subsequently, development and testing of behavioral intervention models to improve adherence and self-management, taking into account issues such as barriers to adherence and psychosocial functioning, will be critical to assuring the efficacy of treatment regimens and health outcomes in this population.
Funding
Food Allergy Project, the Buckeye Foundation and the Campaign Urging Research for Eosinophilic Disease (CURED) Foundation (partial); Center for the Promotion of Treatment Adherence and Self-Management at Cincinnati Children’s Hospital Medical Center (partial).
Conflict of Interest statement: Marc E. Rothenberg MD, PhD, has proprietary interest in reslizumab, a drug being developed by Cephalon. All other authors, none declared.
References
- Bauman L J, Wright E, Leickly F E, Crain E, Kruszon-Moran D, Wade S L, Visness C M. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002;110(1 Pt 1):e6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- DeBrosse C W, Case J W, Putnam P E, Collins M H, Rothenberg M E. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatric and Developmental Pathology. 2006;9(3):210–218. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- Hommel K A, Davis C M, Baldassano R N. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflammatory Bowel Disease. 2009;15(4):589–593. doi: 10.1002/ibd.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel K A, Mackner L M, Denson L A, Crandall W V. Treatment regimen adherence in pediatric gastroenterology. Journal of Pediatric Gastroeneterology and Nutrition. 2008;47(5):526–543. doi: 10.1097/MPG.0b013e318175dda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacouras C A, Furuta G T, Hirano I, Atkins D, Attwood S E, Bonis P A, Burks A W, Chehade M, Collins M H, Dellon E S, Dohil R, Falk G W, Gonsalves N, Gupta S K, Katzka D A, Lucendo A J, Markowitz J E, Noel R J, Odze R D, Putnam P E, Richter J E, Romero Y, Ruchelli E, Sampson H A, Schoepfer A, Shaheen N J, Sicherer S H, Spechler S, Spergel J M, Straumann A, Wershil B K, Rothenberg M E, Aceves S S. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. Journal of Allergy and Clinical Immunology. 2011;128(1):3–20.e26. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: Identifying adolescents' perceptions of barriers to adherence. Journal of Pediatric Psychology. 2003;28(6):383–392. doi: 10.1093/jpepsy/jsg028. [DOI] [PubMed] [Google Scholar]
- Mackner L M, Crandall W V. Oral medication adherence in pediatric inflammatory bowel disease. Inflammatory Bowel Diseases. 2005;11(11):1006–1012. doi: 10.1097/01.mib.0000186409.15392.54. [DOI] [PubMed] [Google Scholar]
- McQuaid E L, Kopel S J, Klein R B, Fritz G K. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. Journal of Pediatric Psychology. 2003;28(5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- McQuaid E L, Walders N, Kopel S J, Fritz G K, Klinnert M D. Pediatric asthma management in the family context: The family asthma management system scale. Journal of Pediatric Psychology. 2005;30(6):492–502. doi: 10.1093/jpepsy/jsi074. [DOI] [PubMed] [Google Scholar]
- Ooi C Y, Bohane T D, Lee D, Naidoo D, Day A S. Thiopurine metabolite monitoring in paediatric inflammatory bowel disease. Alimentary Pharmacology and Therapeutics. 2007;25(8):941–947. doi: 10.1111/j.1365-2036.2007.03278.x. [DOI] [PubMed] [Google Scholar]
- Phares V, Lopez E, Fields S, Kamboukos D, Duhig A M. Are fathers involved in pediatric psychology research and treatment? Journal of Pediatric Psychology. 2005;30(8):631–643. doi: 10.1093/jpepsy/jsi050. [DOI] [PubMed] [Google Scholar]
- Rapoff M A. Adherence to Pediatric Medical Regimens. 2nd ed. New York: Springer; 2010. [Google Scholar]
- Rashid M, Cranney A, Zarkadas M, Graham I D, Switzer C, Case S, Molloy M, Warren R E, Burrows V, Butzner J D. Celiac disease: Evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics. 2005;116(6):e754–759. doi: 10.1542/peds.2005-0904. [DOI] [PubMed] [Google Scholar]
- Varni J W, Seid M, Kurtin P S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Walders N, Kopel S J, Koinis-Mitchell D, McQuaid E L. Patterns of quick-relief and long-term controller medication use in pediatric asthma. Journal of Pediatrics. 2005;146(2):177–182. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Westman E, Ambler G R, Royle M, Peat J, Chan A. Children with coeliac disease and insulin dependent diabetes mellitus–growth, diabetes control and dietary intake. Journal of Pediatric Endocrinology and Metabolism. 1999;12(3):433–442. doi: 10.1515/jpem.1999.12.3.433. [DOI] [PubMed] [Google Scholar]