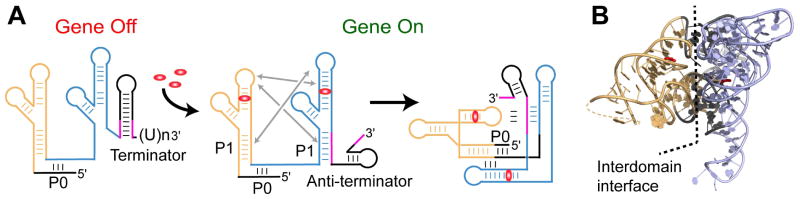
Fig. 4.
Two glycine molecules bind each domain of a tandem glycine riboswitch to elicit a regulatory response. Domains I and II are shown in orange and blue, respectively. A. In the off position, P1 of domain II is not paired, allowing the formation of a terminator at the 3′ end. Upon glycine binding to the riboswitch, a conformational change brings the apical loop of domain I to P1 of domain II, and the apical loop of domain II binds P1 of domain I. Interdomain interactions facilitates glycine binding and stabilize the structure, facilitating formation of the anti-terminator at the 3′ end, and allowing gene transcription to take place. B. A ribbon representation of the X-ray structure (PDB ID 3P49) [43] of the two tandem domains of the glycine riboswitch, with nucleotides stabilizing interactions between domains shown in dark grey.