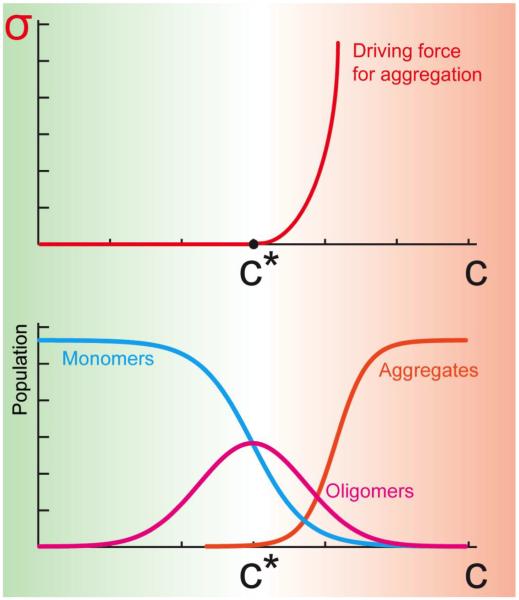
Figure 1. Supersaturation is a major driving force for protein aggregation.
The supersaturation, σ, which can be defined as the ratio between the cellular concentration (C) and the critical concentration (C*) of a protein, represents a driving force for aggregation. Below the critical concentration most proteins are in the monomeric state, with a small fraction in oligomeric states. Above the critical concentration, proteins begin to form insoluble aggregates, including amyloid fibrils, with some remaining fractions in soluble monomeric and oligomeric forms. Thermodynamically, the supersaturation represents the driving force for aggregation associated with the imbalance in the number of molecules in the aggregated and soluble states, and it grows as σ=exp(βΔμ), where Δμ is the difference of the chemical potentials of the aggregated and soluble states, and β=1/kBT.