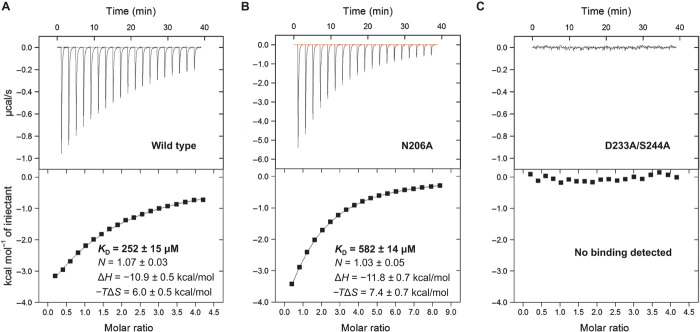
Fig. 5. Binding affinity of wild-type and variant BabA proteins to Leb.
(A and B) Calorimetric response (top) and binding isotherm (bottom) of (A) wild-type BabA titrated with Leb and (B) the BabA N206A variant titrated with Leb. The continuous line in both lower panels represents the least-squares fit of the data to a single-site binding model. The reported thermodynamic parameters are the average (±SEM) of three independent experiments. (C) No calorimetric response (top) or binding isotherm (bottom) was obtained by titrating BabA containing D233A/S244A substitutions with Leb. All calorimetric titrations were performed at pH 7.4.