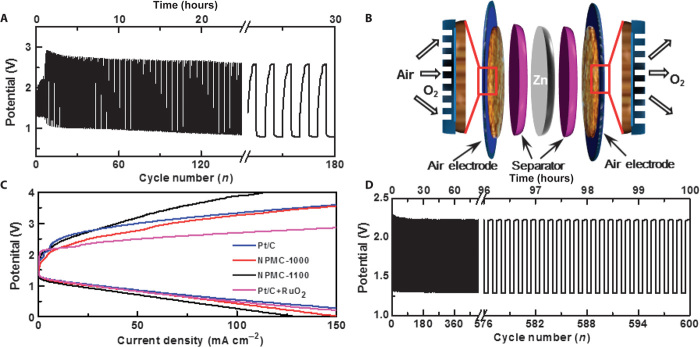
Fig. 11. Performance of rechargeable Zn-air batteries.
(A) Discharge/charge cycling curves of a two-electrode rechargeable Zn-air battery at a current density of 2 mA cm−2 using the NPMC-1000 (pyrolysis at 1000°C) air electrode. Three-electrode Zn-air batteries. (B) Schematic illustration for the basic configuration of a three-electrode Zn-air battery by coupling a Zn electrode with two air electrodes to separate ORR and OER. The enlarged parts illustrate the porous structures of the air electrodes, which facilitates the gas exchange. (C) Charge and discharge polarization curves of three-electrode Zn-air batteries using the NPMC-1000, NPMC-1100, or commercial Pt/C catalyst as both of the air electrodes, along with the corresponding curve (that is, Pt/C + RuO2) for the three-electrode Zn-air battery with Pt/C and RuO2 nanoparticles as each of the air electrodes, respectively. (D) Discharge/charge cycling curves of a three-electrode Zn-air battery using NPMC-1000 as air electrodes (0.5 mg cm−2 for ORR and 1.5 mg cm−2 for OER) at a current density of 2 mA cm−2. [From J. Zhang, Z. Zhao, Z. Xia, L. Dai, A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 10, 444–452 (2015). Reprinted with permission from the Nature Publishing Group.]