Abstract
Mild cognitive impairment (MCI) is associated with early memory loss, Alzheimer neuropathology, inefficient or ineffective neural processing, and increased risk for Alzheimer’s disease (AD). Unfortunately, treatments aimed at improving clinical symptoms or markers of brain function generally have been of limited value. Physical exercise is often recommended for people diagnosed with MCI, primarily because of its widely reported cognitive benefits in healthy older adults. However, it is unknown if exercise actually benefits brain function during memory retrieval in MCI. Here, we examined the effects of exercise training on semantic memory activation during functional magnetic resonance imaging. Seventeen MCI participants and 18 cognitively intact controls, similar in sex, age, education, genetic risk, and medication use, volunteered for a 12-week exercise intervention consisting of supervised treadmill walking at a moderate intensity. Both MCI and control participants significantly increased their cardiorespiratory fitness by approximately 10% on a treadmill exercise test. Before and after the exercise intervention, participants completed a fMRI famous name discrimination task and a neuropsychological battery, Performance on Trial 1 of a list-learning task significantly improved in the MCI participants. Eleven brain regions activated during the semantic memory task showed a significant decrease in activation intensity following the intervention that was similar between groups (p-values ranged .048 to .0001). These findings suggest exercise may improve neural efficiency during semantic memory retrieval in MCI and cognitively intact older adults, and may lead to improvement in cognitive function. Clinical trials are needed to determine if exercise is effective to delay conversion to AD.
Keywords: Alzheimer’s Disease, Dementia, Exercise, Magnetic Resonance Imaging, Non-Pharmacologic Treatment, Physical Activity, Physical Fitness, Memory
Introduction
There is an urgent need to identify effective treatments that may improve cognitive function and brain function in those most at risk for Alzheimer’s disease (AD) [1]. One of the greatest predictors of AD is a diagnosis of mild cognitive impairment (MCI), with 40% of individuals diagnosed with MCI progressing to AD over a 4-year period [2] and approximately 60% exhibiting autopsy-verified AD post-mortem [3]. Recent diagnostic criteria now include the term ‘MCI due to AD’, underscoring the probable decades-long neuropathologic history that eventually leads to clinically observable symptoms and a MCI diagnosis [4, 5]. In addition to impaired episodic memory function, one of the first observable symptoms of MCI due to AD is the inability to remember familiar names, the most common memory complaint among older adults [6]. Indeed, the semantic memory system, in particular, is vulnerable to the earliest stages of AD [7, 8].
In addition to episodic and semantic memory impairments, individuals diagnosed with MCI have been shown to exhibit alterations in cerebral perfusion [9] and functional activation of brain networks [10–12], as well as increased brain amyloid [13, 14], cortical thinning [15], and atrophy of the hippocampus [16]. It has been hypothesized that reduced functional connectivity within the default mode network may be related to increased amyloid retention, and further, that greater task-activated neuronal activity may exacerbate amyloid deposition [17, 18]. Greater task-induced brain activation during successful memory retrieval may reflect a compensatory response [19, 20] or utilization of cognitive reserve [21] that helps to sustain normal function. Alternatively, it is possible that greater task-related neural activation is a sign of underlying neuropathology and neural inefficiency or overload that may be indicative of impending cognitive decline [22, 23]. For example, it has been suggested that a successful intervention for cognitive decline will result in more efficient neural processing, perhaps via reduced neural interference [23], and thus, a reduction in the neural activation required to perform a memory task [10, 19, 20, 23].
Despite the need for effective treatments for early memory loss, a recent NIH consensus panel concluded there was no solid evidence for any intervention or modifiable factor to improve memory or brain function in MCI [24]. It was noted, however, that physical exercise is one intervention that has shown considerable promise. Previous studies in cognitively intact healthy older adults have shown that exercise training [25] or greater self-reported physical activity [26] was associated with an increase or greater fMRI activation during a cognitive task. Although exercise is known to produce cognitive and hippocampal benefits in healthy older adults [27, 28], we know very little regarding how, or if, exercise may affect brain function in patients diagnosed with MCI [1]. Two clinical trials have shown that an exercise intervention leads to limited improvement in cognitive function in MCI participants [29, 30] and older adults with subjective memory complaints [31]. Another study found greater caudate activation during semantic memory retrieval in physically active compared to physically inactive MCI participants [32]. Greater grey matter volume was reported in early-stage AD patients who had greater cardiorespiratory fitness compared to those with lower fitness [33]. However, it is unknown if exercise training alters neural processing during memory retrieval in individuals diagnosed with MCI. The purpose of the current study was to determine if a 12-week walking exercise intervention affects semantic memory fMRI activation and neuropsychological outcomes in individuals diagnosed with MCI compared to cognitively intact older adults. Based on the previous effects of exercise on task-activated fMRI, we hypothesized that exercise training would lead to an increase in semantic memory-related activation in both MCI participants and healthy controls.
Materials and Methods
Participants and Pre-Screening
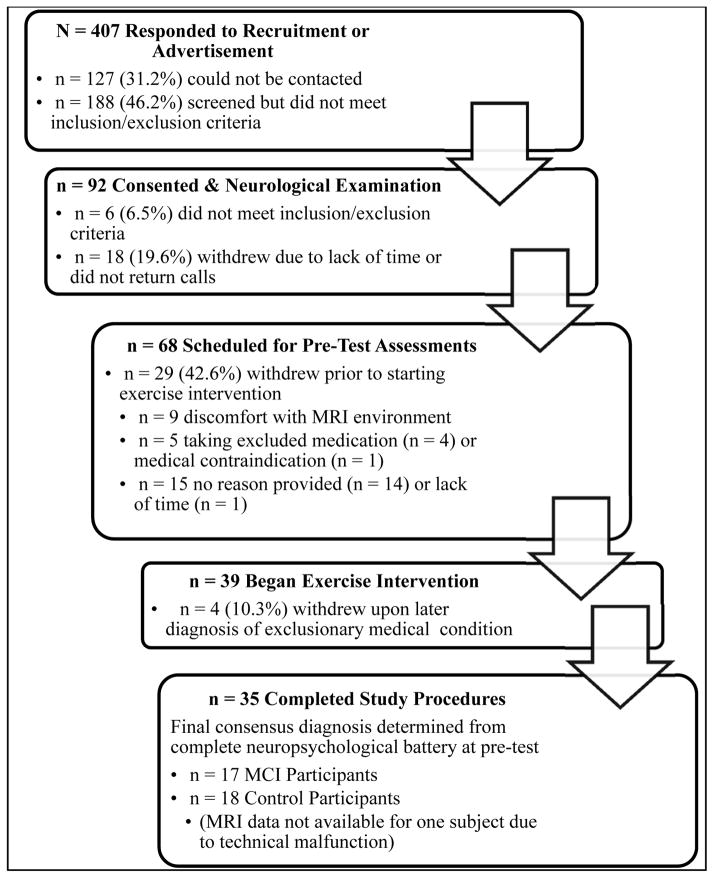
Community dwelling older adults, ages 60 to 88 years, were recruited from in-person informational sessions at retirement communities and community recreation centers, through newspaper and other local advertisements, and through referrals from local physicians. Participants were pre-screened with a structured telephone interview to determine eligibility. Eligible volunteers then provided written informed consent, physician approval for moderate intensity exercise was obtained, and a neurological evaluation was conducted to further determine eligibility. On a separate day, prior to baseline neuropsychological or exercise testing, eligible participants underwent a mock MRI scan session and practiced the fMRI task. Participants who completed the baseline neuropsychological and exercise testing sessions were paid for their participation. Figure 1 describes the flow of participants from initial recruitment to the completion of the study. This study was conducted according to the Helsinki Declaration of 1975 and was approved by the Institutional Review Board at the Medical College of Wisconsin.
Figure 1.
Flow chart of participant recruitment, eligibility screening, enrollment, withdrawals, and the final sample included in the fMRI analysis (n = 34).
Inclusion and Exclusion Criteria
Volunteers who indicated they engaged in less than 3 days/week of moderate intensity physical activity for the past 6 months were included. Participants were excluded if they reported a history or evidence of: 1) neurological illnesses/conditions, such as head trauma with significant loss of consciousness (>30 min), cerebral ischemia, vascular headache, carotid artery disease, cerebral palsy, epilepsy, brain tumor, chronic meningitis, multiple sclerosis, pernicious anemia, normal-pressure hydrocephalus, HIV infection, Parkinson’s disease, or Huntington’s disease; 2) medical illnesses/conditions that may affect brain function, such as untreated hypertension, glaucoma, and chronic obstructive pulmonary disease; 3) current untreated Axis I psychiatric disturbance meeting DSM-IV Axis I criteria, including severe depressive symptoms and substance abuse or dependence; 4) exclusion criteria specific to MR scanning: pregnancy, weight inappropriate for height, ferrous objects within the body, and a history of claustrophobia; 5) left-handedness (laterality quotient [LQ] < 50) [34]; 6) current use of psychoactive medications, except stable doses of SSRI and SNRI antidepressants; 7) any unstable or severe cardiovascular disease or asthmatic condition; and 8) history of transient ischemic attack or > 4 on the modified Hachinski ischemic scale. The Geriatric Depression Scale (GDS) [35] and the Lawton and Brody Self-Maintaining and Instrumental Activities of Daily Living (ADL) Scale [36] were also administered. Participants were excluded if they scored > 15 on the GDS, or showed relatively impaired ADLs. Participants taking acetylcholinesterase inhibitors (AChEI), as well as beta-blockers or other anti-hypertensive medications, were included as long as their medication dosage was stable for at least one month prior to and during the intervention. There were no significant differences between the groups in AChEI use.
Neuropsychological Test Battery and Clinical Criteria for MCI
A comprehensive neuropsychological test battery was administered before and after the exercise intervention, between 0700 hrs and 1100 hrs, prior to the exercise test and on a different day than the MRI scan session. The battery included the Mini-Mental State Exam [37], Mattis Dementia Rating Scale – 2 [38], Rey Auditory Verbal Learning Test (AVLT) [39], the Logical Memory and Letter-Number Sequencing subtests of the Wechsler Memory Scale-III [40], Symbol-Digit Modalities Test [41], Controlled Oral Word Association Test [42], animal fluency, and the Clock Drawing Test [43]. Alternate forms of the AVLT and the DRS were used at the pre- and post-intervention test sessions. Determination of cognitive status (MCI or healthy control) was determined using the core clinical criteria set forth by the recent NIH-Alzheimer’s Association workgroup on the diagnosis of MCI due to AD [4]. MCI was defined by: 1) subjective concern regarding change in cognition; 2) impairment in one or more cognitive domains; 3) preservation of independence in activities of daily living; and 4) not demented.
Exercise Test
Before and after the exercise intervention, participants completed a submaximal exercise test to estimate peak aerobic capacity (VO2peak). Prior to each exercise test, the metabolic cart system was calibrated against known concentrations of O2 and CO2. The exercise test was conducted on a motorized treadmill (General Electric, Milwaukee, WI) using a modified Balke-Ware protocol (2.0 mi/hr (3.2 km/hr) at 0° grade, with grade increase of 1° per minute), in accordance with the American College of Sports Medicine Guidelines [44]. Each test began and ended with 3–5 min of level walking at 1–2 mi/hr (1.6–3.2 km/hr). Heart rate, blood pressure (every 2 minutes), ratings of perceived exertion (every minute), and expired air (VO2, VCO2; every 15 sec) were collected during each test (ParvoMedics, Sandy, UT). The exercise test was terminated when the participant’s heart rate reached 85% of heart rate reserve, if there was an abnormal blood pressure response (e.g., raise in diastolic blood pressure > 110 mmHg), or the participant indicated a desire to stop the test. Heart rate reserve was defined as age-predicted maximal heart rate (220–age) minus resting heart rate determined after 10 minutes of supine rest prior to the exercise test. VO2peak (ml/kg/min at STPD) was estimated from the highest VO2 value obtained during the test [44].
Exercise Intervention
Participants completed a 12-week treadmill walking exercise intervention (44 total sessions). A qualified personal fitness trainer or exercise physiologist supervised each session of exercise, which was conducted individually or in a group of no more than two participants at a fitness center location near their home or within their community. The exercise intensity, session duration, and weekly frequency was gradually increased during the first 4 weeks until the participants were walking 30 minutes per session, 4 sessions per week, at an intensity approximately 50–60% of heart-rate reserve (HRR) during weeks 5–12. The intervention was tailored to each individual based on baseline exercise capacity. The treadmill grade and/or speed were modified each session based on the heart rate and perception of effort of the participant (not more than 15 on the Borg 6–20 RPE scale) and was designed to moderately challenge the participant and to increase cardiorespiratory fitness. Each session began and ended with 10 minutes of very light walking and flexibility exercise. Participants wore a Polar ® heart rate monitor and also provided subjective ratings of perceived exertion throughout each exercise session [45, 46].
Functional MRI Famous Name Recognition Task
Participants underwent an MRI session before and after (within 3–5 days) the intervention on a day that neuropsychological testing or exercise was not performed. The fMRI task stimuli consisted of 30 names of easily recognized famous persons (e.g., Frank Sinatra) and 30 names of non-famous individuals chosen from a local phone book. Only names with a high rate of identification (> 90% correct for targets and foils) were selected from an original pool of 784 names [47]. A trial consisted of the visual presentation of a single name for 4 s. Participants were instructed to make a right index finger key press if the name was famous and a right middle finger key press if the name was non-famous. Both accuracy (% correct) and reaction time (in ms) were recorded. The 60 name trials were randomly interspersed with 20 4-s trials in which the participant was instructed to fixate on a single centrally placed crosshair in order to introduce “jitter” into the fMRI time course. The imaging run began and ended with 12 s of fixation. Total time for the single imaging run was 5 min 44 s.
fMRI Acquisition
Whole-brain, event-related fMRI was conducted on a General Electric (Waukesha, WI) 3.0 Tesla scanner equipped with a quad split quadrature transmit/receive head coil. Images were collected using an echoplanar pulse sequence (TE = 25 ms; flip angle = 77 degrees; field of view (FOV) = 240 mm; matrix size = 64 × 64). Thirty-six contiguous axial 4-mm-thick slices were selected to provide coverage of the entire brain (voxel size = 3.75 × 3.75 × 4 mm). The interscan interval (TR) was 2 s. High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired (TE = 3.9 ms; TR = 9.6 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12 degrees; number of excitations (NEX) = 1; slice thickness = 1.0 mm; FOV = 240 mm; resolution = 256 × 224). Foam padding was used to reduce head movement within the coil.
Image Analysis
During image analysis, the analyst was blind to participant group. Functional images were analyzed with the Analysis of Functional NeuroImages (AFNI) software package [48]. For each image time series, the first 5 TRs were excluded, and each subsequent point was time-shifted to the beginning of the TR. The time series were spatially registered to reduce the effects of head motion, aligned to the participant’s high resolution anatomical image, transformed into standard stereotaxic space [49], spatially smoothed with a 4 mm Gaussian full-width half-maximum filter, and scaled to percent signal change. A deconvolution analysis was used to extract separate hemodynamic response functions (HRFs) for famous and non-famous names from the time-series. HRFs were modeled for the 0–16 s period post-stimulus onset. Motion parameters were incorporated into the model as nuisance regressors. Despite the high task accuracy rate (see Table 2), estimation of the HRFs for identification of famous names and rejection of non-famous names was restricted to correct trials. Area under the curve (AUC) was calculated by summing the hemodynamic responses at time points 4, 6, and 8 s post trial onset.
Spatial Extent Analysis
This analysis was performed to examine between group differences in the spatial extent of activation comparing the Famous and Non-famous name conditions. For each group, statistical parametric maps were generated to identify voxels where the AUC for famous names differed significantly from the AUC for non-famous names. An individual voxel probability threshold (t (16) = 3.25, p = .005) was coupled with a minimum cluster volume threshold of 0.343 ml (8 contiguous voxels). This combination of individual voxel probability and minimum cluster size thresholds is equivalent to a whole brain family-wise error threshold of p < .05 based on 100,000 Monte Carlo simulations [50]. Volume of significant activation was determined for each group at the pre- and post-intervention scans for descriptive comparison; however, the volumes were not statistically compared between groups or across time.
Functional Region of Interest (fROI) Analysis
A fROI analysis was conducted to evaluate potential group differences in the magnitude of the BOLD response in functionally active regions [12, 26, 32]. A fROI map was generated with a disjunction mask by conjoining the activated regions identified in the spatial extent analysis across the four groups. Any voxel deemed significantly activated by the Famous-Non-famous name subtraction in at least one of the four groups contributed to the final fROI map. For each participant, an average AUC was calculated from all voxels within each fROI.
APOE Genotyping
APOE genotype was determined from a venous blood sample using a PCR method described by Saunders et al. [51, 52], with modification. DNA was isolated from 300ul whole blood using the UltraClean Blood DNA Isolation kit (non-spin) (MoBio, Carlsbad, CA), according to manufacturer’s instructions. Isolated DNA (2ul) was amplified with primers specific for APOE2, APOE3, and APOE4 in separate reactions with FAST SYBR Green master mix (Life Technologies, Grand Island, NY) using a StepOne Plus real-time PCR system (Life Technologies). All reactions were examined for amplification and genotype resolved by melt peak analysis.
Statistical Analysis
AUC (4, 6, and 8 s post stimulus onset) in each fROI served as the dependent variable in a 2 Group (MCI vs. Healthy Controls) X 2 Time (Pre-Exercise vs. Post-Exercise) repeated measures analysis of variance (ANOVA) (SPSS 19) to examine main effects of Group and Time, and the interaction between Group and Time. The false discovery rate (FDR) was calculated to control the family-wise error rate for the multiple repeated measures ANOVAs conducted on the fROIs. Similar 2 Group X 2 Time repeated measures ANOVAs were conducted for the neuropsychological test outcomes and measure of cardiorespiratory fitness (VO2peak). Group demographic variables were compared before the exercise intervention using independent samples t-tests. Significance was determined by a two-tailed alpha < .05.
Results
Participant Baseline Characteristics
The MCI and Control groups did not differ prior to the exercise intervention in age, education, sex, proportion of APOE-ε4 carriers, and ADL scores (see Table 1). As expected, the Controls performed significantly better than the MCI participants on all of the neuropsychological measures, except DRS-Construction (see Table 1). The MCI participants had slightly greater symptoms of depression, however the mean scores for both groups were in the normal range [35]. The groups were intact and did not differ on activities of daily living.
Table 1.
Participant demographic data and baseline characteristics.
| Variables | MCI(n=17) Mean(SD) |
Controls(n=18) Mean(SD) |
Group Diff, p |
|---|---|---|---|
| Demographics | |||
| Age (yrs) | 78.7 (7.5) | 76.0(7.3) | .29 |
| Education (yrs) | 15.5 (3.0) | 16.6(2.6) | .22 |
| Sex | 7M, 10F | 3M,15F | .15F |
| AChEI use (#) | 3 | 1 | .34F |
| APOE-ε4 carriers (#) | 6 | 6 | .59F |
| Neuropsychological Testing | |||
| Logical Memory IR | 27.6 (12.5) | 42.5(7.4) | .0001 |
| Logical Memory DR | 16.2 (10.2) | 25.6(6.2) | .002 |
| Logical Memory Recog | 23.1 (3.7) | 26.1(1.9) | .006 |
| DRS Attn | 35.4(1.6) | 36.3(0.8) | .024 |
| DRS I/P | 31.9 (7.0) | 36.6(0.9) | .008 |
| DRS Cons | 5.7 (0.9) | 6.0(0.0) | .15 |
| DRS Conc | 35.5 (4.5) | 38.1(1.1) | .026 |
| DRS Mem | 20.4 (4.5) | 23.8(1.6) | .004 |
| DRS Total | 128.8 (13.3) | 140.5(2.5) | .001 |
| LNS Total | 7.2 (2.9) | 9.6(2.0) | .007 |
| BDS | 17.2 (2.0) | 18.8(0.6) | .003 |
| COWA FAS | 31.2 (13.1) | 41.1(9.2) | .014 |
| Category Fluency - Animals | 13.2 (7.1) | 20.7(4.1) | .0005 |
| Clock Drawing Test | 2.5 (1.2) | 1.4(0.8) | .002 |
| Depression Symptoms and Activities of Daily Living | |||
| GDS | 6.3 (3.6) | 3.4(2.8) | .030 |
| Lawton ADL | 4.7 (0.5) | 4.7(0.5) | .98 |
Note: F = Fisher’s Exact Test; AChEI = acetylcholinesterase inhibitor; APOE-ε4 = apolipoprotein E epsilon 4 allele; Logical Memory = Wechsler Memory Scale-III subtest; IR = immediate recall; DR = delayed recall; Recog = Recognition; DRS = Mattis Dementia Rating Scale-2; Attn = Attention; I/P = Initiation/Perseveration; Cons = Construction; Conc = Concentration; Mem = Memory; LNS = Wechsler Adult Intelligence Scale-III Letter Number Sequencing; BDS = Behavioral Dyscontrol Scale; COWA = Controlled Oral Word Association Test; GDS = Geriatric Depression Scale; ADL = activities of daily living. The mean (SD) scores on the Mini-Mental State Exam, administered to 9 patients and 15 controls, were 24.9 (2.8) and 28.9 (1.1), respectively (p = .00002).
Exercise Intervention Fidelity
The mean (±SD) number of exercise sessions completed, which did not differ between groups, was 42.3 (2.2) out of 44 total sessions, and the mean (±SD) adherence rate was 96.1 (5.0%) of the total exercise sessions. The mean (±SD) intensity of the exercise, which also did not differ between groups, during the first four weeks and weeks 5–12 was 46.9 (7.1%) HRR and 54.7 (11.0%) HRR. The mean (±SD) rating of perceived exertion (RPE) was 10.6 (1.8) and 10.8 (2.0), respectively, which is most closely associated with the verbal descriptor of “Light” exertion. The exercise intervention resulted in a significant mean increase in VO2peak by 2.0 ml/kg/min, an approximately 10.6% increase in cardiorespiratory fitness. Although the MCI group appeared to show a greater increase in VO2peak, the change in cardiorespiratory fitness over time did not significantly differ between the groups (see Table 2), and the groups did not differ in fitness at baseline.
Semantic Memory fMRI Task Behavioral Performance
As shown in Table 2, performance of the famous name recognition task was similar in both the MCI and Control groups; the mean (±SD) percent correct for famous and non-famous names was 80.2 (16.4) and 83.2 (18.6), respectively. The groups did not differ in percent correct famous name recognition or in reaction time for either name category at both pre- and post-exercise intervention, and there were no significant changes over time in task performance in either group. The Control group performed better than the MCI group on percent correct rejection of non-famous names (94.0% vs. 83.6%; p < .05). Only correct trials were included in the analysis of the fMRI data.
Table 2.
Neuropsychological, behavioral, exercise and fMRI task outcome data for both participant groups.
| MCI(n=17) | Controls(n=18) | |||||
|---|---|---|---|---|---|---|
| Variables | Pre-mean (SD) | Post-mean (SD) | Pre-mean (SD) | Post-mean (SD) | Time main effect p | Group x Time effect p |
| Neuropsychological Testing | ||||||
| AVLT Trial 1 | 3.9 (2.1)a | 5.0 (2.1)a | 5.4(1.9) | 6.3 (1.5) | .006 | .80 |
| AVLT Trials 1–5 | 34.9 (13.8) 37.1 (15.5) | 49.3(9.4) | 52.4 (7.9) | .06 | .73 | |
| AVLT IR | 5.1 (4.0) | 5.9 (4.4) | 11.2(2.3) | 11.2 (1.7) | .29 | .29 |
| AVLT DR | 5.5 (4.4) | 5.0 (4.6) | 10.4(3.2) | 11.3 (2.0) | .68 | .23 |
| Clock Drawing Test | 2.5 (1.2) | 2.2 (0.8) | 1.4(0.8) | 1.2 (0.7) | .06 | .49 |
| Depression Symptoms and Physical Function | ||||||
| GDS | 6.3 (3.6) | 6.1 (3.3) | 3.4(2.8) | 3.3 (3.6) | .84 | .94 |
| Lawton ADL | 4.7 (0.5) | 4.5 (0.9) | 4.7(0.5) | 4.7 (0.5) | .23 | .45 |
| Exercise Testing | ||||||
| VO2peak (ml/kg/min) | 19.1 (4.1) | 21.5 (3.4) | 20.1 (5.0) | 21.2 (4.1) | .004 | .24 |
| fMRI Task Performance | ||||||
| % Correct Famous | 80.2 (16.4) | 77.8 (14.0) | 87.1(15.8) | 83.6 (18.9) | .16 | .78 |
| % Correct Non-Famous | 83.2 (18.6) | 84.1 (16.6) | 93.7(6.8) | 94.3 (5.7) | .72 | .93 |
| RT Famous (ms) | 1398 (319) | 1340 (308) | 1335(362) | 1361 (259) | .74 | .41 |
| RT Non-Famous (ms) | 1691 (390) | 1669 (481) | 1574(332) | 1715 (295) | .24 | .12 |
Note: Significant difference between common superscript a, p < .01; ADL = activities of daily living; AVLT = Rey Auditory Verbal Learning Test; DR = delayed recall; GDS = Geriatric Depression Scale; IR = immediate recall; RT = reaction time; VO2peak = peak rate of oxygen consumption. fMRI task performance did not change over time, and the groups differed only in %Correct Non-famous (Group main effect, p < .05).
Semantic Memory fMRI Activation – Spatial Extent Analysis
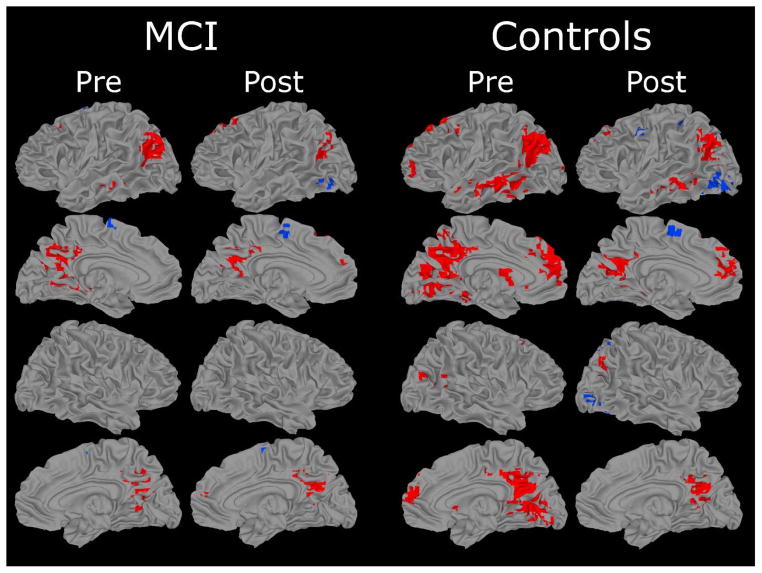
Maps showing regions that were activated in the comparison of famous and non-famous name conditions in the MCI and control groups at the pre- and post- exercise intervention scans are presented in Figure 2 (see Table 3 for activation loci and volumes). The Famous > Non-famous subtraction (shown in red Figure 2) resulted in a greater volume of semantic processing-related activation in the Control group (77.0 ml) compared to the MCI group (19.1 ml) pre-exercise. The volume of activated tissue decreased post-exercise in both groups (to 23.0 ml and 11.3 ml, respectively). Twenty-six regions (i.e., fROIs) showed significant activation in at least one of the groups at one of the time points (shown in Figure 3).
Figure 2.
Results of voxelwise analysis showing brain regions demonstrating significant differences between Famous and Non-Famous name conditions for each group (MCI and Controls). Areas in red indicate Famous > Non-Famous; blue areas indicate Non-Famous > Famous. Location and volume of activation foci delineated in Table 3.
Table 3.
Location and spatial extent of activated regions from a voxel-wise analysis comparing famous versus non-famous name identification. Regions are shown in Figure 2.
| Patients (n = 17) | Controls (n = 17) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Side | Region | BA | Pre | Post | Pre | Post | ||||||||||||
|
| ||||||||||||||||||
| x | y | z | vol | x | y | z | vol | x | y | z | vol | x | y | z | vol | |||
| Famous > Non-Famous | ||||||||||||||||||
| Frontal Lobes | ||||||||||||||||||
| L | MFG | 6 | −28 | 14 | 44 | 0.3 | −37 | 9 | 41 | 0.6 | ||||||||
| L | SFG | 6,8 | −12 | 29 | 50 | 1.7 | −20 | 19 | 53 | 1.6 | −14 | 31 | 47 | 0.6 | ||||
| L | SFG | 6,8 | −29 | 17 | 51 | 0.6 | ||||||||||||
| B | MdFG | 9,10 | 2 | 49 | 19 | 0.6 | −2 | 50 | 25 | 10.9 | −6 | 48 | 22 | 3.6 | ||||
| L | IFG/MFG | 10 | −42 | 45 | 4 | 1.1 | ||||||||||||
| R | MFG/SFG | 8 | 25 | 18 | 47 | 0.6 | ||||||||||||
| L | ACC | 32 | −5 | 26 | 32 | 0.3 | ||||||||||||
| Temporal & Parietal Lobes | ||||||||||||||||||
| B | PCC/Precuneus | 7,18,19, 23,24,29, 30,31 | −2 | −55 | 20 | 9.7 | 0 | −53 | 23 | 6.2 | 0.5 | −57 | 17 | 34.8 | 0.2 | −57 | 21 | 9.9 |
| L | Cuneus | 17,18 | −13 | −96 | 6 | 0.4 | ||||||||||||
| L | STG/MTG | 19,22,39 | −41 | −67 | 24 | 7.1 | −44 | −65 | 24 | 2.2 | −43 | −63 | 27 | 10.9 | −43 | −63 | 28 | 5.4 |
| R | STG | 39 | 54 | −53 | 13 | 0.6 | ||||||||||||
| L | MTG | 20,21, 22,37 | −57 | −35 | −7 | 1.2 | −59 | −31 | −7 | 7.4 | −60 | −34 | −6 | 1.9 | ||||
| R | STG/MTG | 39 | 46 | −71 | 19 | 0.4 | 46 | −68 | 28 | 0.7 | ||||||||
| L | Parahipp/Hipp | −29 | −19 | −11 | 0.4 | |||||||||||||
| R | ParaHipp | 35 | −15 | −15 | 0.5 | 29 | −16 | −15 | 0.3 | |||||||||
| Subcortical and Cerebellum | ||||||||||||||||||
| L | Caudate | −8 | 9 | 10 | 1.9 | |||||||||||||
| R | Caudate/Lent n. | 12 | 12 | 5 | 0.3 | |||||||||||||
| R | Cerebellar Tonsil | 11 | −48 | −38 | 1.2 | |||||||||||||
| R | Inferior Semi-Lunar Lobule | 37 | −71 | −39 | 0.8 | |||||||||||||
| L | Culmen | −12 | −48 | −11 | 0.8 | |||||||||||||
|
| ||||||||||||||||||
| Total Volume Activated (ml): | 19.1 | 11.3 | 77 | 23 | ||||||||||||||
| Non-Famous > Famous | ||||||||||||||||||
| Frontal Lobes | ||||||||||||||||||
| B | SFG/MFG | 6 | −3 | −6 | 61 | 0.7 | −6 | −8 | 51 | 0.4 | ||||||||
| −2 | −6 | 59 | 0.3 | |||||||||||||||
| L | MdFG | 6 | −6 | −4 | 55 | 0.9 | ||||||||||||
| L | MFG/PreG | 6 | −50 | −2 | 41 | 0.6 | ||||||||||||
| Temporal and Parietal Lobes | ||||||||||||||||||
| L | Precuneus/SPL | 7 | −19 | −67 | 40 | 1 | ||||||||||||
| R | Precuneus/SPL | 7 | 30 | −65 | 43 | 0.9 | ||||||||||||
| L | IPL/SMG | 40 | −37 | −38 | 49 | 0.3 | ||||||||||||
| Occipital Lobes | ||||||||||||||||||
| L | LOG/FG | 18,19 | −40 | −67 | −6 | 1.1 | −37 | −72 | −9 | 4.9 | ||||||||
| R | LOG | 18,19 | 36 | −79 | −5 | 1.3 | ||||||||||||
| Subcortical and Cerebellum | ||||||||||||||||||
| L | Lent n./Putamen | −27 | −13 | 7 | 0.4 | |||||||||||||
| −23 | 4 | 13 | 0.3 | |||||||||||||||
|
| ||||||||||||||||||
| Total Volume Activated (ml): | 0.7 | 1.8 | 0 | 10.6 | ||||||||||||||
Note: positive = right (x), anterior (y), and superior (z), representing center of mass in Talairach coordinates; ACC = anterior cingulate cortex; AG = angular g.; BA = Brodmann area; FG = fusiform g.; g. = gyrus; Hipp = hippocampus; IFG = inferior frontal g.; IOG = inferior occipital g.; IPL = inferior parietal lobule; ITG = inferior temporal g.; Lent = Lentiform; LG = lingual g.; LOG = lateral occipital g.; MdFG = medial frontal g.; MFG = middle frontal g.; MOG = middle occipital g.; MTG = middle temporal g.; n. = nucleus; SFG = superior frontal g.; Parahipp = parahippocampal g.; PCC = posterior cingulate cortex; PreG = precentral g.; SMA = supplementary motor area; SMG = supramarginal g.; SPL = superior parietal lobule; STG = superior temporal g.; vol = volume in ml.
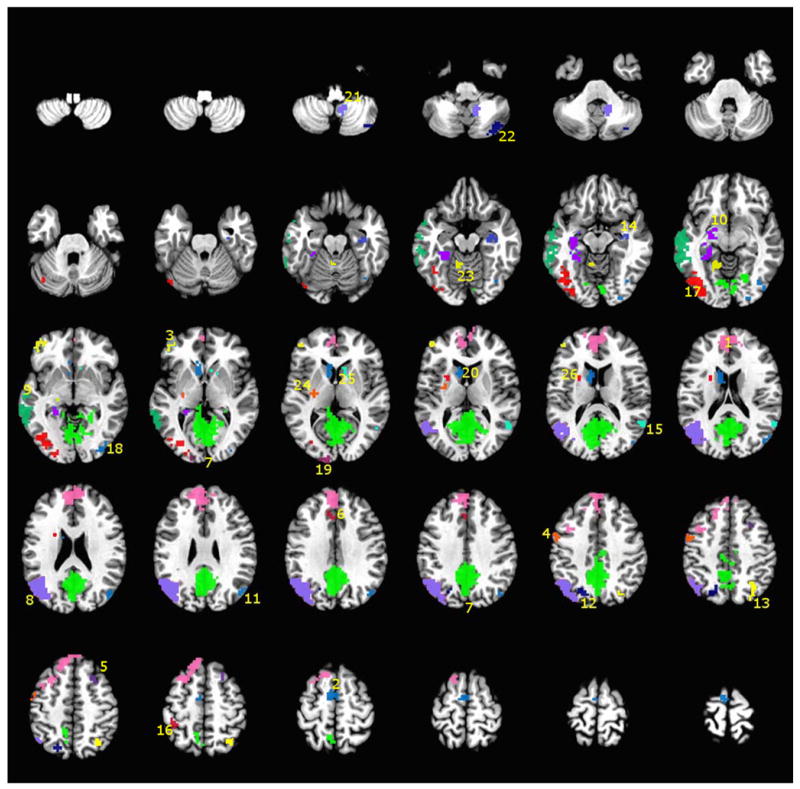
Figure 3.
A montage of axial slices showing the 26 functional regions of interest (fROIs) derived from a disjunction of the regions activated in both the MCI and Control groups at both the pre- and post-exercise intervention fMRI sessions. The numerical labels correspond to the region numbers shown in Table 4 and Figure 4. The colors only denote the spatial location of the distinct fROIs, which may appear in multiple slices of the montage.
Semantic Memory fMRI Activation – fROI Analysis
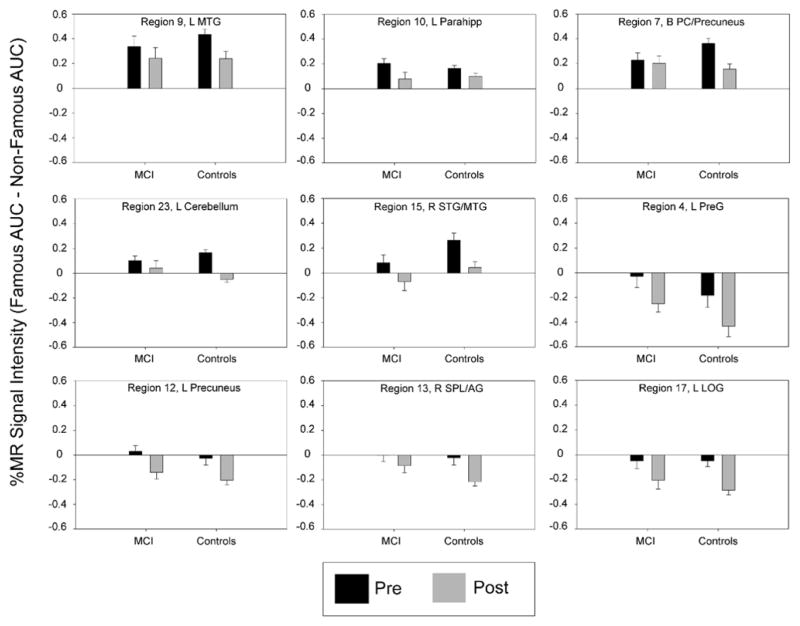
The analysis of mean activation intensity (%AUC) in the 26 fROIs revealed significant main effects of Time in 11 regions, significant main effects of Group in three regions, and significant Group × Time interactions in three regions (see Table 4). Seven regions survived the FDR threshold among the main effects of Time; however, none of the main effects for Group or the Group × Time interactions survived the FDR threshold. Figure 4 shows the seven fROIs that survived the FDR threshold for the main effect of Time, plus two additional regions with p-values that were just above the FDR threshold. In all regions and in both groups, semantic memory-related activation significantly decreased from pre- to post-exercise intervention.
Table 4.
Twenty-six functional regions of interest derived by a disjunction of the voxel-wise maps of the two groups at the pre- and post-intervention scans, compared using a 2(Group) X 2(Time) ANOVA on the mean percent area under the curve of each region.
| Region # | Side | Region Label | BA | x | y | z | vol | Time p | Time η2 | Group p | Group η2 | G × T p | G × T η2 | Group Pre p | Group Post p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Lobes | |||||||||||||||
| 1 | B | MdFG/SFG | 6,8,9,10 | −8 | 41 | 31 | 16.0 | ns | --- | ns | --- | ns | --- | 0.0429 | ns |
| 2 | B | MdFG/SMA | 6 | −5 | −6 | 57 | 1.8 | ns | --- | ns | --- | ns | --- | ns | ns |
| 3 | L | MFG | 10 | −42 | 45 | 4 | 1.1 | ns | --- | ns | --- | ns | --- | ns | ns |
| 4 | L | PreG | 6 | −49 | −2 | 41 | 0.6 | 0.0091 | 0.194 | ns | --- | ns | --- | ns | ns |
| 5 | R | MFG/SFG | 8 | 25 | 18 | 46 | 0.6 | ns | --- | ns | --- | ns | --- | 0.0213 | ns |
| 6 | L | ACC | 32 | −5 | 26 | 32 | 0.3 | ns | --- | 0.010 | 0.192 | ns | --- | 0.0038 | ns |
| Temporal & Parietal Lobes | |||||||||||||||
| 7 | B | PC/Precuneus | 7,18,19,23,24,29,30,31 | 1 | −57 | 18 | 38.0 | 0.0241 | 0.149 | ns | --- | ns | --- | ns | ns |
| 8 | L | MTG/AG | 19,22,39 | −41 | −63 | 26 | 12.9 | 0.0395 | 0.126 | ns | --- | ns | --- | ns | ns |
| 9 | L | MTG | 20,21,22,37 | −58 | −31 | −7 | 8.2 | 0.0230 | 0.151 | ns | --- | ns | --- | ns | ns |
| 10 | L | Lent n./Parahipp | 20,36,37 | −25 | −28 | −8 | 3.2 | 0.0022 | 0.257 | ns | --- | ns | --- | ns | ns |
| 11 | R | MTG | 39 | 45 | −69 | 25 | 1.1 | ns | --- | ns | --- | ns | --- | ns | ns |
| 12 | L | Precuneus | 7 | −19 | −67 | 40 | 1.0 | 0.0006 | 0.314 | ns | --- | ns | --- | ns | ns |
| 13 | R | SPL/AG | 7 | 30 | −65 | 43 | 0.9 | 0.0047 | 0.224 | ns | --- | ns | --- | ns | ns |
| 14 | R | Hipp | 33 | −15 | −15 | 0.8 | ns | --- | ns | --- | ns | --- | ns | ns | |
| 15 | R | STG/MTG | 39 | 54 | −53 | 13 | 0.6 | 0.0003 | 0.346 | ns | --- | ns | --- | 0.0376 | ns |
| 16 | L | IPL/SMG | 40 | −37 | −38 | 49 | 0.3 | ns | --- | ns | --- | 0.024 | 0.150 | ns | 0.0134 |
| Occipital Lobes | |||||||||||||||
| 17 | L | LOG | 18,19 | −37 | −72 | −6 | 5.4 | 0.0006 | 0.310 | ns | --- | ns | --- | ns | ns |
| 18 | R | LOG | 18,19 | 35 | −79 | −5 | 1.3 | 0.0481 | 0.117 | ns | --- | ns | --- | ns | ns |
| 19 | L | MOG | 17,18 | −13 | −96 | 6 | 0.4 | ns | --- | ns | --- | ns | --- | ns | ns |
| Subcortical and Cerebellum | |||||||||||||||
| 20 | L | Caudate | −8 | 10 | 10 | 1.9 | ns | --- | ns | --- | ns | --- | 0.043 | ns | |
| 21 | R | Cerebellum | 11 | −48 | −38 | 1.2 | ns | --- | ns | --- | 0.029 | 0.140 | ns | ns | |
| 22 | R | Cerebellum | 36 | −70 | −39 | 0.8 | ns | --- | ns | --- | 0.038 | 0.127 | ns | ns | |
| 23 | L | Cerebellum | −11 | −47 | −12 | 0.8 | 0.0001 | 0.374 | ns | --- | 0.018 | 0.164 | ns | ns | |
| 24 | L | Lent n./Putamen | −27 | −13 | 7 | 0.4 | ns | --- | 0.011 | 0.186 | ns | --- | ns | 0.004 | |
| 25 | R | Caudate | 12 | 13 | 5 | 0.3 | ns | --- | ns | ns | ns | --- | ns | ns | |
| 26 | L | Lent n./Putamen | −23 | 4 | 14 | 0.3 | ns | --- | 0.018 | 0.164 | ns | --- | ns | 0.015 | |
Note: All p-values shown in bold font exceeded the False Discovery Rate (FDR) threshold to control for family-wise error from multiple fROI comparisons. Regions 7 & 9 (p-values in italics) just missed the FDR threshold and are included in Figure 4. The Group and Group X Time effects did not survive the FDR threshold. Region # corresponds to labels in Figure 3; η2 = partial eta-squared; positive = right (x), anterior (y), and superior (z), representing center of mass in Talairach coordinates; ACC = anterior cingulate cortex; AG = angular g.; BA = Brodmann area; FG = fusiform g.; g. = gyrus; Hipp = hippocampus; IFG = inferior frontal g.; IOG = inferior occipital g.; IPL = inferior parietal lobule; ITG = inferior temporal g.; Lent = Lentiform; LG = lingual g.; LOG = lateral occipital g.; MdFG = medial frontal g.; MFG = middle frontal g.; MOG = middle occipital g.; MTG = middle temporal g.; n. = nucleus; SFG = superior frontal g.; Parahipp = parahippocampal g.; PCC = posterior cingulate cortex; PreG = precentral g.; SMA = supplementary motor area; SMG = supramarginal g.; SPL = superior parietal lobule; STG = superior temporal g.; vol = volume in ml.
Figure 4.
Percent magnetic resonance (MR) signal intensity (Famous AUC minus Non-Famous AUC) in nine fROIs that showed a significant main effect of Time based on a 2 (Group) × 2 (Time) repeated measures ANOVA. All regions, except regions 7 & 9, exceeded the False Discovery Rate threshold to control for family-wise error from multiple fROI comparisons. Region numbers and labels correspond to those provided in Table 4 and Figure 3.
Neuropsychological Test Performance
As shown in Table 2, Trial 1 learning on the AVLT significantly improved from pre- to post-exercise intervention in both the MCI and Control groups. There was also nearly significant improvement on Trials 1–5 learning on the AVLT (p = .06) and the Clock test (p = .06). With the exception of ADLs, which were intact and did not differ between groups, the MCI participants and controls differed significantly on the neuropsychological diagnostic and outcome measures before and after the intervention, and there were no significant Group × Time interactions for the neuropsychological outcomes.
Discussion
A 12-week walking exercise intervention led to a 10% increase in maximal aerobic capacity and an associated decrease in semantic memory retrieval-related fMRI activation in MCI participants, and cognitively intact older adults. Single trial list-learning significantly improved in both groups, and learning through repetition (AVLT Trial 1–5 sum) improved by approximately two words in the MCI participants and by approximately three words in cognitively intact elders. While these cognitive improvements did not differ statistically between the groups, the quality of the cognitive improvement in MCI participants was remarkable given their history of cognitive decline and likelihood for future cognitive decline. Despite the use of alternate forms, some improvement due to familiarity with the test format might be expected in cognitively intact participants. Yet, the familiarity effect in persons with impaired cognition is typically observed as a stable performance over time. Although the controls continued to outperform MCIs, the cognitive improvement in this MCI sample exceeds what could be expected with repeated test administration [53]. While we did not have a non-exercise control condition, this observation nonetheless represents a sizeable treatment effect in the context of baseline performance. Indeed, the achievement of cognitive stability in MCI is considered a marker of treatment success in pharmacologic [54], cognitive training [55], and exercise interventions [31]. The cognitive improvement in the MCI participants in the current study is supported by the changes in task-activated fMRI that were concurrently observed. This supports the hypothesis that exercise may impact on the neural networks related to memory retrieval.
Our hypothesis of increased fMRI activation after the exercise intervention was not confirmed. We based our prediction on the few previous studies among healthy older adults that reported increased task-activated fMRI activation after exercise training or in more physically active participants. In cross-sectional comparisons of healthy APOE-ε carriers vs. non-carriers, physically active ε4 carriers showed greater fMRI activation [26] and were less likely to exhibit cognitive decline than those who were less active or non-carriers [56]. In the only other exercise intervention to examine effects on task-activated fMRI, cognitively intact older adults exhibited improved inhibitory control (flanker) task performance and, as a consequence of the intervention, increased fMRI activation in middle and superior frontal gyri and the superior parietal lobule, and decreased activation in the anterior cingulate cortex [25]. Thus, because the intervention led to both a change in behavioral task performance and currently recorded neural activation, the effects of the intervention on brain networks are difficult to isolate. As such, these results are difficult to compare with the current study where task performance was constant. In our study, behavioral performance on the alternate forms of a famous name task was always high in both groups, did not change over time, and we only included correct trials in the fMRI analysis. Thus, we examined the effects of exercise on successful semantic memory retrieval at both the pre- and post-intervention measurements, without the confounding effects of improved task performance. Given the consistent memory retrieval performance, our results suggest that semantic memory retrieval-related neural activation became more efficient in both MCI and cognitively intact participants from before to after the exercise intervention.
These effects highlight one of the primary theoretical debates in the fields of cognitive aging and dementia. While greater neural activation during memory retrieval may reflect successful compensation or neural reserve, as suggested for example by the STAC theory [19], compensation resulting in greater neural activation is also paradoxically associated with increased brain amyloid accumulation and greater probability of MCI diagnosis. This suggests the compensatory response, while promoting a preservation of function, may ultimately lead to greater AD pathology. Our previous cross-sectional [26, 32] and longitudinal work [56] is consistent with the idea that greater neural activation, as measured by greater extent and intensity of fMRI activation during fame recognition, is associated with cognitive stability over time. However, our current prospective intervention data suggest that exercise training may enhance cognitive and neural reserve in MCI, not through a greater capacity to activate neural tissue, as we initially predicted, but rather through a reduced neural workload during successful engagement of semantic memory networks. Whether or not exercise training alters the underlying clinical or neuropathological trajectory of AD remains to be determined.
We selected a semantic memory fMRI task as the primary outcome. While previous studies have shown that fMRI activation during episodic memory tasks may predict future cognitive decline [11, 57], others have reported that semantic memory fMRI activation may be better predictors of longitudinal cognitive change than episodic memory fMRI tasks [58]. The famous name recognition task we used has several advantages over episodic memory encoding or recognition memory tasks. First, the famous name task requires little effort and both memory impaired and cognitively intact persons can perform the task with a high degree of accuracy. Moreover, the event-related fMRI design permits the exclusion of incorrect trials and thus limits the analysis to only successful memory performance. Blocked fMRI designs, for example the comparison of a memory encoding block to a rest block [11], do not allow one to distinguish correct from incorrect task performance and thus groups differences may reflect activation due to greater effort or task difficulty in the cognitively impaired group. The high performance in both groups within the current study, paired with the inclusion of only correct trials, removes any confounding influence of task difficulty or performance differences between groups. Moreover, famous name task performance (by design) did not change over time in either the MCI or cognitively intact elders. Thus, the effects we observed cannot be attributed to improved task performance. Rather, our results suggest exercise training resulted in a reduced neural response to perform consistently successful semantic memory retrieval, an effect observed in both controls and MCI participants.
One interesting exception to this effect occurred in the precuneus and posterior cingulate cortex (PCC). These regions, which show a reduction in BOLD signal intensity during non-semantic tasks compared to the resting state [59], are activated by a number of semantic tasks [60]. The precuneus and PCC activation in the current study, along with the other regions activated by the famous name task, overlaps with the semantic memory system and the ‘default mode network’, consistent with data indicating the resting state reflects ongoing semantic processing [60]. Importantly, these are regions that show hyperactivation and resistance to deactivation during non-semantic tasks in MCI, and also exhibit early signs of amyloid retention in both MCI and healthy APOE-e4 carriers [10]. As MCI progresses to AD, precuneus/PCC hypometabolism and reduced functional connectivity have been observed [17, 18]. One hypothesis is that precuneus/PCC hyperactivation in MCI is a compensatory response to hippocampal neurodegeneration [10, 17, 19]. In our sample, while the Group × Time interaction in the bilateral precuneus/PCC was not significant (region 7 in Figure 4, p = .08), an exploratory post-hoc analysis showed that the decrease over time was significant in the control group (p = .002), but not in the MCI group (p > .7). It is possible that the effects of exercise training on precuneus/PCC activation were blunted in the MCI participants in order to preserve compensatory activation in the face of early hippocampal neurodegenerative processes. This is consistent with our previous study that found no difference in precuneus/PCC activation during fame discrimination between physically active and physically inactive MCI participants [32]. In the cognitively intact controls, there may have been room to improve on the efficiency in the precuneus/PCC region because it was not necessary for this region to simultaneously compensate for medial temporal lobe dysfunction. However, this interpretation is speculative and should be viewed with caution, as we did not measure AD-related neuropathology in our participants.
Previous exercise clinical trials in MCI participants have been limited to outcomes from neuropsychological testing [1]. Two studies that reported a failure of improvement in neurocognitive performance in MCI after an exercise intervention suffer from somewhat questionable efficacy of the intervention [61, 62]. In contrast, another study reported improved verbal fluency, Stroop Color-Word Interference and Symbol-digit Modalities Test performance after a 6-month exercise intervention, which evidenced a comparable increase in fitness as achieved in the current study (11.5% vs. 10.6% in the current study) [29]. In contrast to the current study, their effects were observed only in women, whereas we did not find a difference between men and women on any outcome. Finally, a study of elders with MCI and elders with subjective memory complaints not meeting criteria for MCI demonstrated stable cognition in those who participated in a 6-month home-based exercise program, compared with those who did not exercise, who declined significantly over the same period (change of −0.26 points, 95% CI −0.89–0.54) [31]. These results also add support for our interpretation of the current study that improved cognition in MCI over a 3-month exercise intervention is remarkable.
Memory training interventions have demonstrated effectiveness to improve training-related and non-training-related cognitive task performance in healthy younger and cognitively intact older adults [63]. The changes in task-related fMRI activation after memory training interventions, however, have been inconsistent, with some studies reporting decreased activation and some increased activation [63]. In one study, fMRI activation increased in both older adults diagnosed with MCI (mean age 70 years) and healthy controls after a 6-week, one session per week, memory training intervention [64]. That study employed an episodic memory task in a blocked fMRI design, where task performance differed between groups and both groups exhibited task improvement after training. As with some exercise interventions, this leaves unclear the foundations for activation changes. In the current study, our MCI and control participants did not differ or change over time on the activation task and only correct trials were included in the analysis, thereby precluding the likelihood that the effects could be due to inherent group differences or task difficulty. Moreover, despite the primary effect of reduced magnitude of semantic memory activation, MCI participants also showed new areas of activation after the intervention (see Table 3, Figure 2), particularly in left superior frontal gyrus, medial frontal gyrus (Brodmann’s areas 6 & 8), and left lateral occipital and fusiform gyri (Brodmann’s areas 18 & 19). These results are consistent with the findings of Belleville et al. and partly support theories that hypothesize cognitive improvement or compensation will result in the recruitment of new neural circuits [20, 21, 65]. Although the comparative and potential synergistic effects of exercise with cognitive training interventions are of great interest [56], direct comparisons of the combined treatments have not yet been made.
One limitation of this study is that we did not include a ‘no treatment’ or active control condition. Thus, we are not able to directly account for the effects that may be due to the passage of time or practice. Some have suggested that repeated fMRI scans result in reduced task activation due to familiarity with the task or scanner environment [66]. However, such fMRI practice effects also are accompanied by moderate to large task-related learning effects from the first to the second session. Thus, it is possible the fMRI changes over time reflect changes in task difficulty, which could have resulted in greater error (in blocked designs) or effort-related activation on the first scan. Yet, when a well-learned task is repeated after several weeks, the changes in fMRI activation are very minimal and bidirectional [67]. This is a critical distinction when making comparisons across studies [58]. Our famous name recognition task is not subject to task-related practice effects. The participants enter the scanner with decades of experience recognizing famous names and so perform the task with ease, even with existing episodic memory impairments. Moreover, task performance did not improve over time. We also have shown (unpublished data), using the same famous name task in a sample of 16 cognitively intact APOE-ε4 carriers, that the patterns and intensity of fMRI activation did not change over an 18-month interval during which little or no regular physical activity occurred, in the absence of any intervention [68]. In regard to changes over time on the AVLT, stable cognitive function among healthy older adults is often denoted by a small increase in cognitive test performance on alternate forms due to familiarity with the test format [69]. Among those with MCI, this familiarity effect often manifests as equivalent performance, not increased performance, over repeated test administrations [53]. Thus, given the expectation that those with MCI, in the absence of intervention, are likely to exhibit cognitive decline, the measured cognitive improvement in the MCI group suggests a real treatment effect that can be attributed to the exercise intervention. We acknowledge, however, that additional randomized controlled trials are needed.
We used a sound experimental manipulation of cardiorespiratory fitness in response to a well-supervised and well-attended exercise intervention to determine if neural function during memory retrieval could be affected in participants with MCI. Our results provide the first evidence that exercise may induce neural plasticity in individuals diagnosed with MCI. However, the rigor necessary to achieve a similar degree of integrity in an exercise intervention may not be feasible in larger clinical trials, and thus the effects we report may be attenuated in community-based or unsupervised exercise programs. Another limitation is that our sample was primarily Caucasian and well educated, and so it will be important to determine the effects of exercise in multiple ethnic groups. Lastly, our MCI group was by definition not demented and had intact ADLs. These effects may not generalize to individuals diagnosed with AD, or to those with greater clinical symptoms or functional impairments.
Regarding potential mechanisms, one can only speculate due to the pleiotropic effects of exercise. In rodents, exercise has been well documented to stimulate the transcription, translation, and release of neurotrophic factors (e.g., brain derived neurotrophic factor, insulin-like growth factor-1) and to promote neurogenesis, particularly in the dentate gyrus of the hippocampal formation [70–72]. There is also preliminary evidence that exercise may produce these neurotrophic effects in the hippocampal formation in healthy adults [73] and healthy older adults [27]. It is also possible that the cholinergic effects of exercise may increase cerebral perfusion, possibly affecting the neurodynamics of the blood-oxygen level dependent fMRI signal [74] that may reflect improved network efficiency [23]. There is preliminary evidence that exercise may attenuate the accumulation of beta-amyloid in rodents [75] and cognitively intact older adult APOE-ε4 carriers [76]. However, it is unknown if these potential mechanisms are engaged by exercise in individuals diagnosed with MCI or others at increased AD risk [1].
The current findings are consistent with the effects of greater left caudate activation in more physically active MCI participants [32]. Dopaminergic projections within frontal-striatal networks, which are agonized by exercise [77] and by working memory training interventions [78], may dampen neural interference and improve the gain on task relevant neural signals during semantic memory retrieval [23, 79]. Moreover, noradrenergic activation in response to single sessions of exercise has been shown to benefit memory consolidation in MCI [80], suggesting that the long-term cognitive benefits may accumulate with repeated bouts of exercise.
In conclusion, a 12-week walking exercise intervention in MCI participants resulted in improved single trial list-learning performance and a reduction in fMRI activation during a semantic memory retrieval task. Decreased fMRI semantic memory-related activation after the exercise intervention, coupled with improved episodic memory performance, suggests exercise training may improve neural efficiency during successful memory retrieval in MCI. This further suggests the large-scale manipulations of cardiovascular and brain neurotrophic systems with exercise promote neural plasticity and improved brain function in MCI. Whether or not these effects can delay or prevent AD conversion, and how exercise compares to pharmacologic, cognitive training, or other interventions, remains to be determined.
Acknowledgments
We thank all of the participants for their time and effort, and gratefully acknowledge the cooperation and collaboration of the community and commercial fitness centers where the participants exercised. We also appreciate the dedication of our excellent research staff, including study coordinator Karen Outzen; exercise physiologists and personal fitness trainers Andrew Mattes, Karlee Sweere, Lynn Wheeler, Rebecca Ohmen, Bethany Brooks, Ed Possing, Bradley Kohl, David Cornell, Jacqueline Geib, David Johnson, Rong Tang, and William Massey; and the nurses and MRI technicians at the CTSI Translational Research Unit at the Medical College of Wisconsin. Additionally, we acknowledge Kevin Regner, M.D., Jeanne Palmer, M.D., Elizabeth Cogbill, M.D., Tinoy Kizhakekuttu, M.D., and Kodlipet Dharmashankar, M.D., for their collegial provision of medical coverage during exercise and MRI test sessions; and Erin Browning for her technical expertise and help in MRI data collection. Technical support for APOE genotyping was provided by Stacy Meyer and Erin Koester. This work was supported by a grant from the Graduate School Research Growth Initiative at the University of Wisconsin-Milwaukee; and by the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health [grant number UL1RR031973].
References
- 1.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Rao SM. Physical activity and brain function in older adults at increased risk for Alzheimer’s disease. Brain Sciences. 2013;3:54–83. doi: 10.3390/brainsci3010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 3.DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 4.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Lonie JA, Herrmann LL, Tierney KM, Donaghey C, O’Carroll R, Lee A, Ebmeier KP. Lexical and semantic fluency discrepancy scores in aMCI and early Alzheimer’s disease. J Neuropsychol. 2009;3:79–92. doi: 10.1348/174866408X289935. [DOI] [PubMed] [Google Scholar]
- 8.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Xu G, Antuono PG, Jones J, Xu Y, Wu G, Ward D, Li SJ. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007;69:1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]
- 10.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, Laviolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional Alterations in Memory Networks in Early Alzheimer’s Disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- 14.Quigley H, Colloby SJ, O’Brien JT. PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry. 2011;26:991–999. doi: 10.1002/gps.2640. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 17.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 22.Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 23.Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- 24.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. NIH State-of-the-Science Conference Statement: Preventing Alzheimer’s Disease and Cognitive Decline. NIH Consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- 25.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, Butts AM, Hantke NC, Lancaster MA, Rao SM. Interactive effects of physical activity and APOE-epsilon4 on BOLD semantic memory activation in healthy elders. Neuroimage. 2011;54:635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etnier JL. Chronic exercise and cognition in older adults. In: McMorris T, Tomporowski PD, Audiffren M, editors. Exercise and Cognitive Function. Wiley-Blackwell; Chichester: 2009. pp. 227–248. [Google Scholar]
- 29.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of Neurology. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, Uemura K, Lee S, Park H. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12:128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Journal of the American Medical Association. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 32.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Verber MD, Durgerian S, Antuono P, Butts AM, Hantke NC, Lancaster MA, Rao SM. Does physical activity influence semantic memory activation in amnestic mild cognitive impairment? Psychiatry Res. 2011;193:60–62. doi: 10.1016/j.pscychresns.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 36.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2 professional manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 39.Rey A. L’examen clinique en psychologie. Presses Universitaires de France Paris; 1964. [Google Scholar]
- 40.Wechsler D. WAIS-III/WMS-III technical manual. Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 41.Smith AR. Symbol Digit Modalities Test. Western Psychological Services; Los Angeles: 1991. [Google Scholar]
- 42.Benton AL, Hamsher K. Iowa City, IA: 1978. [Google Scholar]
- 43.Cosentino S, Jefferson A, Chute DL, Kaplan E, Libon DJ. Clock drawing errors in dementia: neuropsychological and neuroanatomical considerations. Cogn Behav Neurol. 2004;17:74–84. doi: 10.1097/01.wnn.0000119564.08162.46. [DOI] [PubMed] [Google Scholar]
- 44.ACSM. American College of Sports Medicine Guidelines for Exercise Testing and Prescription. Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 45.Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1998. [Google Scholar]
- 46.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exerc. 1997;29:999–1012. doi: 10.1097/00005768-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao SM. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 49.Talairach J, Tournoux P. A co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- 50.Ward BD. Analysis of Functional NeuroImages (AFNI) software. NIH; Bethesda, MD: 2000. [Google Scholar]
- 51.Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, Hyman BT, Crain B, Tang MX, Phelps CH. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med. 1998;338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 52.Saunders AM, Hulette O, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, Alberts MJ, Strittmatter WJ, Breitner JC, Rosenberg C. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer’s disease. Lancet. 1996;348:90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- 53.Devanand DP, Liu X, Brown PJ, Huey ED, Stern Y, Pelton GH. A two-study comparison of clinical and MRI markers of transition from mild cognitive impairment to Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:483469. doi: 10.1155/2012/483469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farlow MR. Treatment of mild cognitive impairment (MCI) Curr Alzheimer Res. 2009;6:362–367. doi: 10.2174/156720509788929282. [DOI] [PubMed] [Google Scholar]
- 55.Stott J, Spector A. A review of the effectiveness of memory interventions in mild cognitive impairment (MCI) Int Psychogeriatr. 2011;23:526–538. doi: 10.1017/S1041610210001973. [DOI] [PubMed] [Google Scholar]
- 56.Woodard JL, Sugarman MA, Nielson KA, Smith JC, Seidenberg M, Durgerian S, Butts A, Hantke N, Lancaster M, Matthews MA, Rao SM. Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Curr Alzheimer Res. 2012;9:436–446. doi: 10.2174/156720512800492477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien JL, O’Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hantke N, Nielson KA, Woodard JL, Breting LM, Butts A, Seidenberg M, Carson Smith J, Durgerian S, Lancaster M, Matthews M, Sugarman MA, Rao SM. Comparison of semantic and episodic memory BOLD fMRI activation in predicting cognitive decline in older adults. J Int Neuropsychol Soc. 2013;19:11–21. doi: 10.1017/S1355617712000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 60.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scherder EJ, Van Paasschen J, Deijen JB, Van Der Knokke S, Orlebeke JF, Burgers I, Devriese PP, Swaab DF, Sergeant JA. Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment Health. 2005;9:272–280. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- 62.Miller LA, Spitznagel MB, Busko S, Potter V, Juvancic-Heltzel J, Istenes N, Glickman E, Gunstad J. Structured exercise does not stabilize cognitive function in individuals with mild cognitive impairment residing in a structured living facility. Int J Neurosci. 2011;121:218–223. doi: 10.3109/00207454.2010.546537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Dev Cogn Neurosci. 2012;2(Suppl 1):S167–179. doi: 10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belleville S, Clement F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134:1623–1634. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- 65.Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 66.Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- 67.Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test--retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001;21:592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 68.Smith JC, Durgerian S, Woodard JL, Nielson KA, Butts AM, Hantke NC, Seidenberg M, Lancaster MA, Matthews MA, Sugarman MA, Rao SM. Physical activity and brain function in older adults at genetic risk for Alzheimer’s disease. Med Sci Sports Exerc. 2012;44:S104. [Google Scholar]
- 69.Shapiro DM, Harrison DW. Alternate forms of the AVLT: a procedure and test of form equivalency. Arch Clin Neuropsychol. 1990;5:405–410. [PubMed] [Google Scholar]
- 70.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. doi: 10.1016/j.nbd.2012.06.011. in press. [DOI] [PubMed] [Google Scholar]
- 73.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Science USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith JC, Paulson ES, Cook DB, Verber MD, Tian Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: implications for fMRI. J Neurosci Methods. 2010;191:258–262. doi: 10.1016/j.jneumeth.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 75.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Castro JM, Duncan G. Operantly conditioned running: effects on brain catecholamine concentrations and receptor densities in the rat. Pharmacology Biochemistry & Behavior. 1985;23:495–500. doi: 10.1016/0091-3057(85)90407-1. [DOI] [PubMed] [Google Scholar]
- 78.Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Neely AS, Virta J, Laine M, Rinne JO. Effects of working-memory training on striatal dopamine release. Science. 2011;333:718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- 79.Crosson B, Benjamin M, Levy I. Role of the basal ganglia in language and semantics: supporting cast. In: Hart J Jr, Kraut MA, editors. Neural Basis of Semantic Memory. Cambridge University Press; New York: 2007. pp. 219–243. [Google Scholar]
- 80.Segal SK, Cotman CW, Cahill LF. Exercise-induced noradrenergic activation enhances memory consolidation in both normal aging and patients with amnestic mild cognitive impairment. J Alzheimers Dis. 2012;32:1011–1018. doi: 10.3233/JAD-2012-121078. [DOI] [PMC free article] [PubMed] [Google Scholar]