ABSTRACT
Relaxin hormone secreted into the circulation during pregnancy was discovered through its effects on pubic symphysis relaxation and parturition. Genetic inactivation of the relaxin gene or its cognate relaxin family peptide receptor 1 (RXFP1) in mice caused failure of parturition and mammary nipple enlargement, as well as increased collagen fiber density in the cervix and vagina. However, the relaxin effect on discrete cells and tissues has yet to be determined. Using transgenic mice with a knockin LacZ reporter in the Rxfp1 allele, we showed strong expression of this gene in vaginal and cervical stromal cells, as well as pubic ligament cells. We produced a floxed Rxfp1 allele that was used in combination with the Tagln-cre transgene to generate mice with a smooth muscle-specific gene knockout. In pregnant females, the ROSA26 reporter activated by Tagln-cre was detected in smooth muscle cells of the cervix, vagina, uterine artery, and in cells of the pubic symphysis. In late pregnant females with conditional gene ablation, the length of pubic symphysis was significantly reduced compared with wild-type or heterozygous Rxfp1+/− females. Denser collagen content was revealed by Masson trichrome staining in reproductive tract organs, uterine artery, and pubic symphysis. The cervical and vaginal epithelium was less developed than in heterozygous or wild-type females, although nipple size was normal and the dams were able to nurse their pups. In summary, our data indicate that relaxin/RXFP1 signaling in smooth muscle cells is important for normal collagen turnover and relaxation of the pubic symphysis during pregnancy.
Keywords: conditional knockout, female reproductive tract, parturition, pubic symphysis, RXFP1, relaxin
INTRODUCTION
Relaxin hormone was first identified by its effects on relaxation of the pubic symphysis in nonpregnant guinea pigs [1]. The process is crucial for passage of the fetus during parturition. Early experiments with injections of relaxin antibody to pregnant female rats and subsequent studies of relaxin (Rln1) and relaxin receptor (Rxfp1) gene knockouts revealed a dystocia phenotype associated with delay and increase of parturition time, and in some cases the complete inability of RLN antibody-treated or mutant females to deliver pups [2–5]. Further studies in some, but not all, species showed that RLN1 contributes to the quiescence of myometrial contractile activity, growth of the uterine endometrium and myometrium, growth and extensibility of the cervix and vagina, proliferation of luminal epithelium in these organs, and many other actions during pregnancy (reviewed in Bathgate et al [6] and Sherwood [7]). In animals lacking Rln1 or Rxfp1, many of these gestational changes were attenuated because of impaired remodeling of extracellular matrix in the reproductive tract tissue; in particular, loosening, realignment, and other changes in collagen fibers and the corresponding increase in tissue water content.
The immunohistochemical expression of the relaxin receptor and radiolabeled relaxin-binding sites were detected in several cell types within the reproductive system, making it difficult to define which targets are responsible for the various effects of relaxin. Moreover, there are significant discrepancies in RXFP1 expression patterns in the reproductive tract, as revealed by different methods [5]. The experiments with LacZ reporter knocked-in Rxfp1 alleles showed prominent gene expression in smooth muscle cells in reproductive organs, and in the cells of the lamina propria, a loose connective tissue beneath the epithelial cell layer [3, 4]; however, it remains unclear whether low-level receptor expression detected in other cells might also contribute to the relaxin-deficient phenotype. Additionally, it was demonstrated that the relaxin effects on stromal cells in the vagina and cervix may mediate proliferative effects in epithelial cells, suggesting a complex system of paracrine signaling between neighboring cells [8].
Apart from its effects on myometrial smooth muscle cells, relaxin has a distinct role in the remodeling of the uterine vasculature during pregnancy. Relaxin increases uterine blood flow and decreases uterine artery stiffness associated with increased expression of elastin, increased collagen density, and lower expression of several metalloproteinases and cell adhesion molecules [9, 10]. Immunohistochemical RXFP1 expression was also detected in vascular smooth muscle cells. It was suggested that decreased fetal weight detected in late-pregnant relaxin-deficient female mice might be related to reduced uterine artery blood flow and compromised uteroplacental perfusion [10]. However, no changes in placental weight were detected in pregnant relaxin knockout females.
During pregnancy the mouse pubic symphysis undergoes dramatic remodeling to allow fetuses safe passage through the birth canal. Pubic symphysis fibrocartilage is gradually replaced by a fibrous connective tissue, creating a flexible interpubic ligament with significant amounts of newly synthesized elastic fibers [11]. In rodents, circulating relaxin increases dramatically during the 2–3 days before delivery [5]. Knockout of the relaxin gene results in the failure of pubic symphysis dilation and an increase of collagen density within the ligament. Significantly, cells of the pubic symphysis show several features of myofibroblasts and express several smooth muscle-specific markers [12].
Here we describe the production of two new alleles of the Rxfp1 gene: a knockin allele with a LacZ reporter inserted into the Rxfp1 locus, thus disrupting the expression of the gene, and the Rxfp1 conditional floxed allele. We used the knockin allele to establish Rxfp1 expression patterns in late-pregnant females and the floxed allele to inactivate the gene in smooth muscle cells expressing Cre recombinase transgene under the control of the mouse transgelin (smooth muscle protein 22-alpha) promoter. Our data indicate that conditional inactivation of the gene affects dilation of the pubic symphysis, vaginal and cervical epithelium development, and collagen density in the female reproductive tract and uterine artery.
MATERIALS AND METHODS
Production of New Alleles of Rxfp1 and Mouse Breeding
All animal studies were approved by the Institutional Animal Care and Use Committees at Florida International University and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The C57BL/6 embryonic stem cells with a targeted Rxfp1 allele were obtained from the EuCOMM/EuMMCR collection. The recombinant allele contains an frt-flanked LacZ/neo cassette, making it a LacZ-knockin, null allele of the gene (Rxfp1tm1a[EUCOMM]Hmgu, Rxfp1LacZ hereafter). Chimeric mice were produced at the University of Texas Health Science Center-Houston, Transgenic Mouse and Stem Cells Core Facility. Confirmation of germ line transmission of the mutant allele was determined with PCR using genomic DNA isolated from ear samples with primers specific for LacZ: LacZF, CAGACGATGGTGCAGGATAT; and LacZR, ATACAGCGCGTCGTGAT. Mice with the floxed Rxfp1 allele (Rxfp1fl) were produced by breeding Rxfp1LacZ females with ROSA-FLP (Gt[ROSA]26Sortm1[FLP1]Dym/RainJ) transgenic mice obtained from The Jackson Laboratory (Fig. 1). The PCR primers used for the genotyping of Rxfp1fl allele were: FP, CATCTGACATGGGAGTGGAA; and RP1, CAGCTGGTGGTGACTGGTTA. Excision of the floxed fourth exon resulted in a deleted allele, Rxfp1Δ, which was detected by PCR using primers FP and RP2, GGCGAGCTCAGACCATAACT. The latter combination of primers did not detect the wild-type allele. Conditional inactivation of Rxfp1 gene was achieved by crossing Rxfp1fl mice with Cre recombinase transgenic mice, driven by the smooth muscle-specific mouse transgelin (smooth muscle protein 22-alpha) promoter Tagln-cre (Tg [Tagln-cre] 1Her/J), also called Sm22a-Cre, obtained from The Jackson Laboratory. Animal genotyping was performed using cre primers creF, ATCAACGTTTTCTTTTCGG; and creR, ATTTGCCTGCATTACCGGTC; and the primers for different Rxfp1 alleles. Mice with knockout/LacZ-knockin allele of Rxfp1, Rxfp1ko, were generated previously in our laboratory [3]. All mice produced in this project or used in crosses were on the C57BL/6 genetic background.
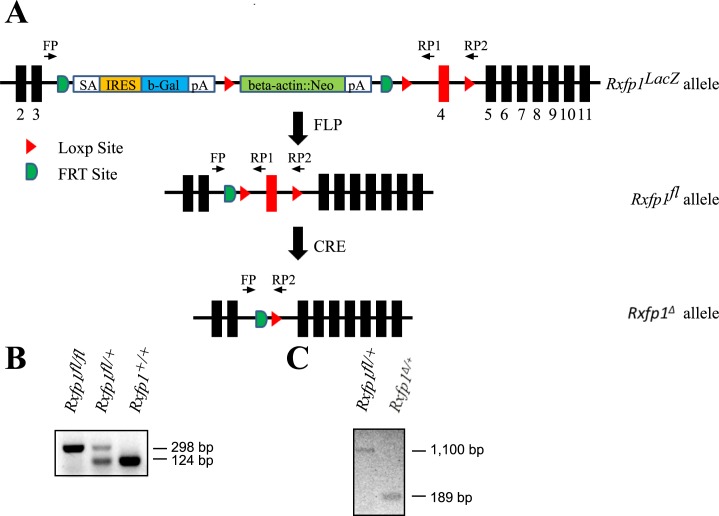
FIG. 1.
LacZ knockin and floxed alleles of Rxfp1. A) Schematic representation of Rfxp1LacZ, Rxfp1fl, and Rxfp1Δ alleles. Exons are shown as black boxes and marked by a number underneath. SA is a splicing acceptor; IRES is an internal ribosome entry site; b-Gal is a β-galactosidase gene; pA is a poly(A) signal; beta-actin::Neo is neomycine-resistant gene driven by β-actin promoter; FLP is flp recombinase; CRE is cre recombinase. There are three LoxP sites for Cre recombinase on the 5′ and 3′ ends of the neo cassette and within intron 4. Rxfp1fl allele is produced by flp-induced recombination, and the deleted allele without exon 4 is produced by cre-induced recombination as shown. The position of the primers used for genotyping is shown with arrows. B) Detection of Rxfp1fl floxed allele with primers FP/RP1. The floxed allele produced a 298-bp band and the wild-type allele produced a 124-bp band. C) Detection of Rxfp1Δ deleted allele with FP/RP2 primers. The floxed allele produced a 1100-bp band and the deleted allele produced a 189-bp band. The wild-type allele is not detectable with this primer pair.
Analysis of Pregnant Females
The Rxfp1fl/+, Tagln-cre/+ females were crossed to Rxfp1ko/Rxfp1ko males to produce mice with a combination of wild-type, floxed allele and knockout Rxfp1 alleles and Cre transgene. Two groups of females from such crosses were used in these experiments: heterozygotes (Rxfp1ko/+ with or without Tagln-cre) and conditional mutants (Rxfp1fl/Rxfp1ko, Tagln-cre). Homozygous females Rxfp1ko/Rxfp1ko of the same age were used as global gene knockout group and Rxfp1fl/Rxfp1fl as wild-type group. The effects of conditional inactivation of Rxfp1 were studied in late pregnant females at 18.5 days after vaginal plug detection. Females were killed using CO2 overexposure. Maternal body weight, litter size, and the weights of viable fetuses were measured. Fetal weight was calculated from the average weights of each litter. Cervical length was measured longitudinally from the vagina to the bifurcation of the uterus in intact females. All reproductive organs were then collected and fixed. The length of the pubic symphysis was determined using a dissecting microscope equipped with an ocular micrometer [13]. The number of animals analyzed is indicated in the figure legends. Differences were expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance with Bonferroni multiple comparison test in GraphPad software.
Histology
Adult female mouse organs were harvested and fixed in 4% paraformaldehyde, washed with PBS, stored in 70% ethanol at 4°C overnight, embedded in paraffin, and sectioned at 6 or 7 μm using standard protocols. Slides were stained using Masson Trichrome Stain kit (Sigma-Aldrich) according to the standard procedure as per manufacturer protocol, with minor adjustments to incubation times. The thickness of cervical and vaginal epithelial cell layers was measured using AxioVision Software (Carl Zeiss Microscopy) on Carl Zeiss Axio A1 Microscope equipped with an AxioCam MRc5 CCD camera in three to four females per group in 10 random cross sections for each sample.
β-Galactosidase Staining
Tissue samples were frozen in OCT solution and 15-μm cryosections were prepared. Slides were fixed in 0.2% paraformaldehyde for 10–15 min on ice. After washing twice in PBS on ice for 10 min, sections were stained using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology) according to the manufacturer's protocol. Slides were washed in PBS, counterstained with eosin, and examined under the Axio A1 microscope. At least three animals were used for each genotype.
RESULTS
Production of Rxfp1LacZ and Rxfp1fl alleles
As other clones in the EUCOMM collection, the Rxfp1 mutant allele contains two FTR sites flanking a LacZ gene with an internal ribosome entry site (IRES) and a neomycin resistance (neo) cassette (Fig. 1), representing the knockout first reporter approach: the transcription of LacZ mRNA matches the expression of Rxfp1 gene, whereas the expression of endogenous full-length Rxfp1 mRNA is disrupted (Fig. 1A). Germ line transmission of the targeted allele from chimeric males was established, and the Rxfp1LacZ mutant line was produced. We produced mice heterozygous for the Rxfp1LacZ allele and previously generated Rxfp1− knockout [3] allele. Males of both genotypes were fully fertile, whereas all females had abnormal parturition; often they were not able to deliver pups, and all had underdeveloped nipples and did not feed their progeny. Thus, the new Rxfp1 allele was not able to complement the knockout allele, indicating the correct targeting of the Rxfp1 locus. To produce the Rxfp1fl mice, we bred the Rxfp1LacZ mice with a transgenic mouse line containing a ubiquitously expressed FLP recombinase; the LacZ/neo cassette was then removed by flippase-induced recombination (Fig. 1A). Females that were homozygous for Rxfp2fl/fl or Rxfp1fl/Rxfp1LacZ were fully fertile with no indication of abnormal parturition or nipple development, suggesting that the floxed Rxfp1fl allele was fully functional. Combination of the allele with Cre transgene led to the recombination of the Rxfp1fl allele and deletion of exon 4, leading to a reading frame shift and premature stop codon in Rxfp1Δ allele (Fig. 1B).
Pattern of Rxfp1 and Tagln-cre Transgene Expression in the Female Reproductive Tract
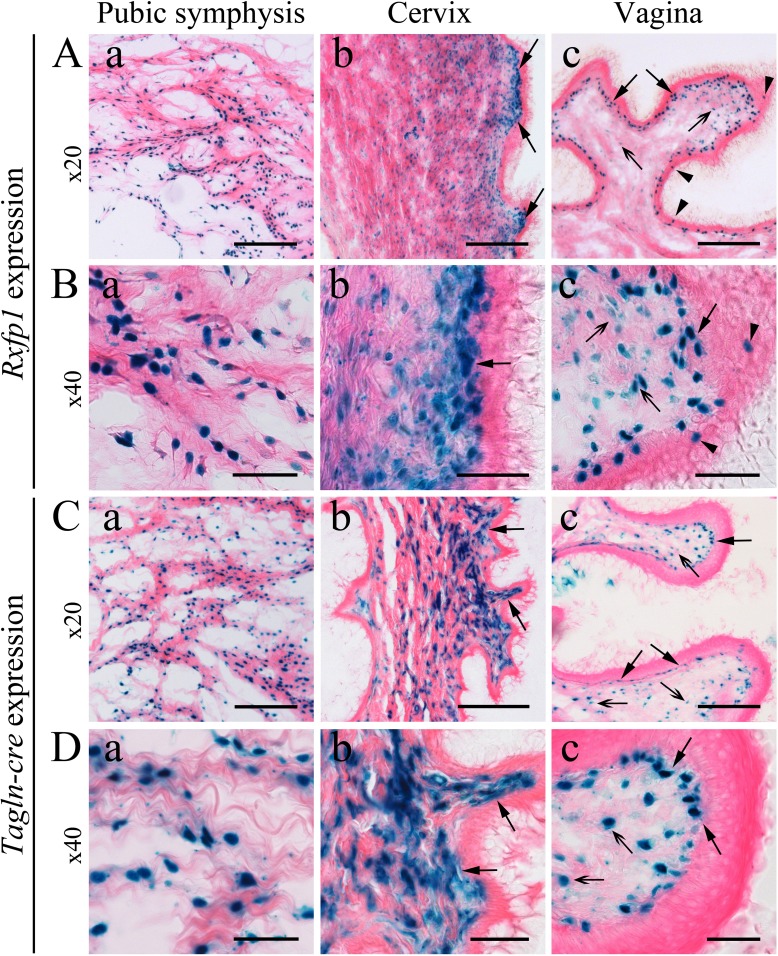
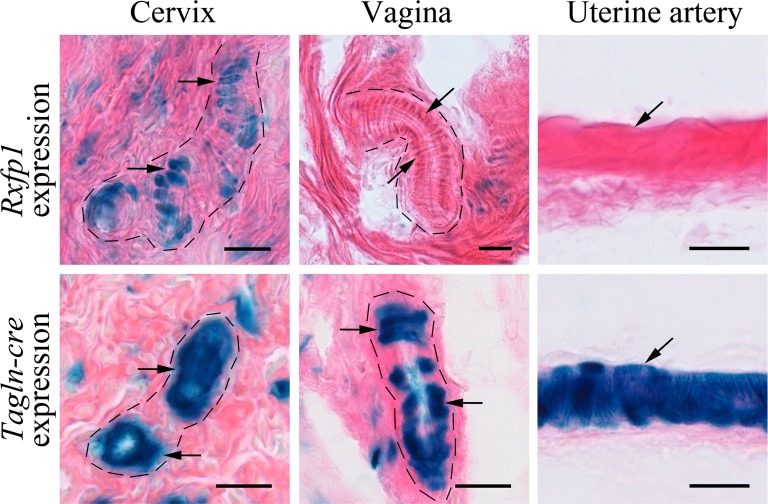
The Rxfp1LacZ/+ heterozygous females with wild-type phenotype and normal development of the reproductive organs were used for analysis. The cervix, vagina, pubic symphysis, and uterine artery were isolated from pregnant females at Day 18.5 after detection of vaginal plugs, and β-galactosidase activity was examined on frozen sections. Consistent with previously published results from two other independent mutants with the Rxfp1 knockin LacZ reporter allele [3, 4], strong staining was revealed in all reproductive organs analyzed (Fig. 2). In the cervix, the Rxfp1 expression was localized in what histologically appears to be smooth muscle cells within the stroma and in cells of the lamina propria underneath the basal layer of epithelium. No staining was detected in the cervical epithelium. In the vagina, most of the staining was localized in the lamina propria, with some weaker staining detected inside the stroma. Single intensely stained cells were present in the layers of vaginal epithelium. Intense staining was detected in all cartilage cells located between the collagenous elastic fibers of the pubic symphysis, especially on the edges of pubic bones. Although in the cervix the vascular smooth muscle cells were stained, no staining was detected in blood vessels in the vagina (Fig. 3). No staining was detected in uterine artery walls under the conditions tested (Fig. 3).
FIG. 2.
Rxfp1 expression and Tagln-cre-mediated recombination of ROSA26 reporter in reproductive organs of late pregnant females. A and B) Representative images of β-galactosidase staining of Rfxp1LacZ/+ female reproductive organs at Day 18.5 of pregnancy. Blue staining labeled by arrows or arrowheads shows gene expression. Aa and Ba) All cartilage cells of the pubic symphysis are strongly positive. Ab and Bb) In the cervix there is strong staining in the lamina propria (arrows) and stromal smooth muscle cells. Ac and Bc) In the vagina there is strong staining in the lamina propria (arrows) and in a few epithelial cells (arrowheads), and some weak staining in the vaginal stroma (arrows with open arrowheads) is shown. C and D) Representative images of β-galactosidase staining of ROSA26-LacZ, Tagln-cre female reproductive organs at Day 18.5 of pregnancy. Ca and Da) Intense staining is located in cells of the pubic symphysis. Smooth muscle cells of the cervix (Cb and Db) and vaginal stromal cells (arrows with open arrowheads; Cc and Dc) are strongly positive, as are the cells of the lamina propria (arrows). At least three mice of each genotype were analyzed. Bars = 100 μm (A and C) and 50 μm (B and D).
FIG. 3.
Rxfp1 expression and Tagln-cre-mediated recombination of ROSA26 reporter in blood vessels. Representative images of β-galactisodase staining of Rfxp1LacZ/+ and ROSA26-LacZ, Tagln-cre blood vessels at Day 18.5 of pregnancy. Blue staining shows gene expression. The dotted line marks the blood vessels within the cervix and vagina. Strong Rxfp1 staining (arrows) was detected in vascular smooth muscle cells in cervical but not in vaginal blood vessels. No staining was detected in the uterine artery. The Tagln-cre transgene induced recombination and expression of LacZ reporter in all vascular smooth muscle cells. At least three mice of each genotype were analyzed. Bars = 20 μm.
As Rxfp1 expression was observed in smooth muscle cells, we used the Tagln-cre transgene for conditional targeting of the gene in cells of smooth muscle lineage in various organs. To establish the pattern of Cre transgene-induced recombination in female reproductive organs, we crossed them with the ROSA26-LacZ reporter mouse line and analyzed Tagln-cre, ROSA26 females at day 18.5 of pregnancy (Fig. 2). Overall, there was a clear overlap of the Tagln-cre staining pattern with Rxfp1 gene expression detected in smooth muscle cells in female reproductive tract organs. As with Rxfp1, the Cre transgene expression was present in smooth muscle cells of the cervix and somatic cells of the vagina as well as in cells of the lamina propria (Fig. 2). The cartilage cells of the pubic symphysis were strongly positive. Prominent expression of the Tagln-cre transgene was present in vascular smooth muscle cells in the uterine artery and blood vessels within the vagina and cervix (Fig. 3). No endogenous β-galactosidase activity was detected in any of the tissues from wild-type Rxfp1+/+ pregnant females under the conditions used for the assay.
Abnormal Development of the Pubic Symphysis in Pregnant Tagln-cre, Rxfp1fl/fl Females
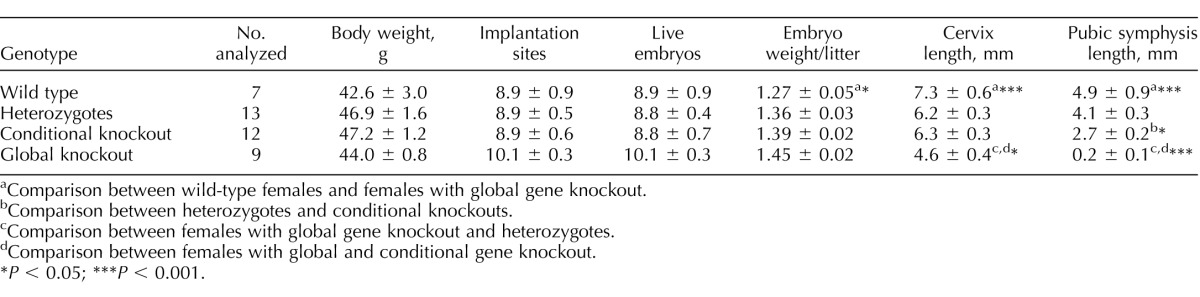
Late pregnancy in conditional Rxfp1 knockout females was chosen as a point of analysis based on the previously described abnormalities in mice with global Rln1 and Rxfp1 gene deletion. To increase the probability of detecting the phenotype in the conditional mutants, we produced mice heterozygous for knockout allele mutation, Rxfp1ko, floxed allele Rxfp1fl, and Tagln-cre. Heterozygous Rxfp1ko/+ mice with and without Cre transgene derived from the same crosses were used as controls. Additionally, Rxfp1fl/fl and Rxfp1ko/ko females of the same age were used as the wild-type and global gene knockout groups. All mice were on the C57BL/6 background, enabling direct comparisons of the two latter groups with heterozygotes and conditional mutants. The data presented in Table 1 show the reproductive characteristics of four female cohorts. There was no difference in total body weight of the females, number of implantation sites, or live embryos. A small but statistically significant increase in the embryo weight per litter was detected in the global gene knockout versus the wild-type group. The cervical length in global knockout mutants was significantly shorter than in the other three groups, and although there was a decrease in cervical length in heterozygotes or conditional mutants versus wild type, it was not statistically significant. No difference was detected between heterozygotes and conditional mutants in cervical length. Finally, all four groups significantly differed in the length of the pubic symphysis, where the wild type had the longest and the global knockout mutants had the shortest length of the ligament (Fig. 4 and Table 1). The conditional deletion of Rxfp1 led to a 33% decrease in pubic symphysis length during late pregnancy versus heterozygotes. Females with conditional deletion of Rxfp1 delivered and then fed their pups. No postnatal lethality was detected in any litters delivered by conditional knockout females. The milk was clearly visible in their stomachs. The length of mammary nipples in conditional females did not appear altered.
TABLE 1.
Reproductive characteristics of late pregnant females (Day 18.5) with mutations of the Rxfp1 gene.
Comparison between wild-type females and females with global gene knockout.
Comparison between heterozygotes and conditional knockouts.
Comparison between females with global gene knockout and heterozygotes.
Comparison between females with global and conditional gene knockout.
P < 0.05; ***P < 0.001.
FIG. 4.
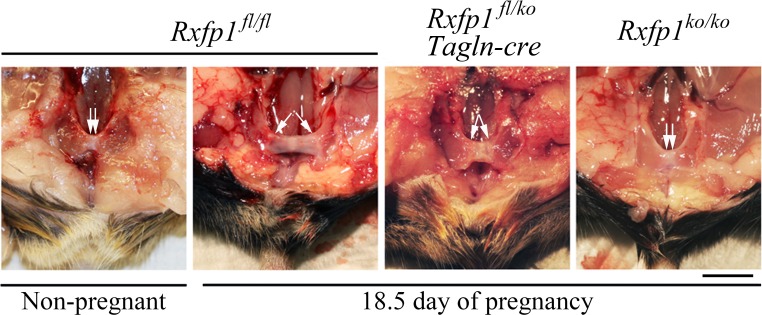
Pubic symphysis elongation in late pregnant females with mutations of Rxfp1 gene. Shown are representative images of dissected pubic symphyses with arrows showing their lengths. Significant relaxation of the pubic symphysis is present in late pregnant versus nonpregnant wild-type females. There was no dilation of the pubic symphysis in Rxfp1ko/ko females and a partial dilation in Rxfp1fl/ko, Tagln-cre females with conditional gene deletion. A total of 6–10 mice of each genotype were analyzed (Table 1). Bar = 5 mm.
Changes in Histological Organization of the Reproductive Tract
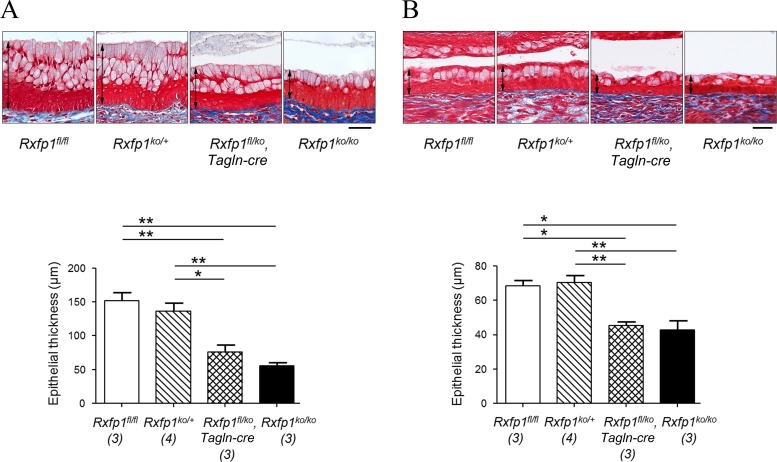
Relaxin deficiency in Rln1−/− females and in rats treated with antibodies to relaxin resulted in the decreased length of vaginal and cervical epithelium [5, 14, 15]. In our experiments, in wild-type homozygotes the thickness of cervical and vaginal epithelium was significantly higher than in global knockout mutants or in heterozygotes (P < 0.05 for cervical epithelium; P < 0.01 for vaginal epithelium; Fig. 5). In conditional mutants the thickness of cervical and vaginal epithelium was comparable with that of knockout mutants and was significantly lower than that of wild-type or Rxfp1ko/+ heterozygotes.
FIG. 5.
Cervical and vaginal epithelium in late pregnant females with mutations of the Rxfp1 gene. Representative images of Masson trichrome staining of vaginal (A) and cervical (B) epithelium in wild-type (Rxfp1fl/fl), heterozygous (Rxfp1ko/+), conditional (Rxfp1fl/ko, Tagln-cre), and global (Rxfp1ko/ko) knockout females at Day 18.5 of pregnancy. The thickness of the epithelium was measured in three to four females per group. Significant differences between groups are indicated as *P < 0.05; **P < 0.01 (ANOVA with Bonferroni multiple comparison test). Bar = 50 μm.
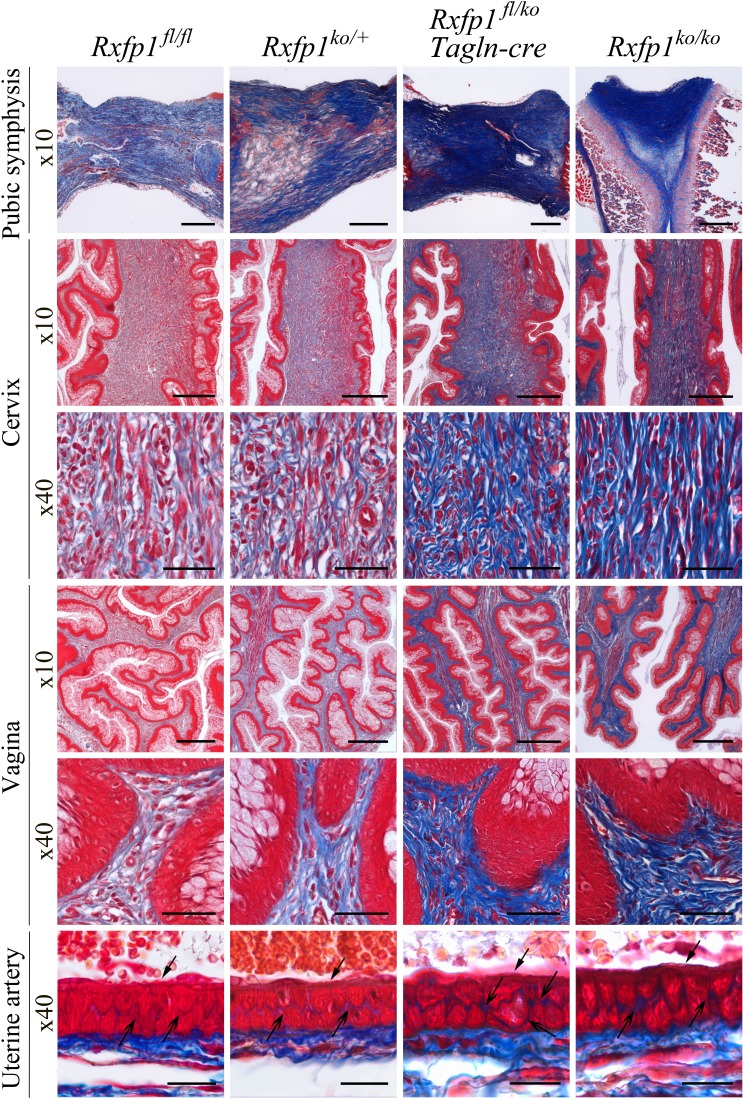
It was also reported that in late pregnant Rln1-deficient females the collagen content in stromal tissues was denser than in wild-type controls [14, 15]. Similarly densely stained collagen was observed in mutant pubic symphyses. We used Masson trichrome staining of the vagina, cervix, pubic symphysis, and uterine artery to visualize these changes (Fig. 6). In all samples collagen was located in the stromal compartment of the tissues. Comparison of the cervix and vagina isolated from wild-type and knockout pregnant females confirmed a looser arrangement of collagen fibers in wild-type than in Rxfp1−/− animals. Ablation of relaxin signaling resulted in an increase in collage density of the vaginal and cervical stroma. The luminal lining of the stratified squamous epithelium in the global gene knockout cervix and vagina was visibly thinner than in wild-type controls. In heterozygotes, the collagen staining in the vagina and cervix had the same intensity as in the wild-type group, whereas the conditional knockouts had staining similar to the global knockouts. There was also a clear increase in collagen staining between smooth muscle cells of the uterine artery in both conditional and global gene knockouts compared with heterozygous or wild-type controls. Finally, dense collagen staining was detected in cross sections through the pubic symphysis in conditional and global gene knockouts. Heterozygous pubic symphyses had “wild-type”-like loose collagen fiber arrangements; however, some denser areas stained dark blue were also present (Fig. 6).
FIG. 6.
Increased collagen density in late pregnant females with global and smooth muscle-specific deletions of the Rxfp1 gene. Masson trichrome staining was used to visualize collagen organization within the cervix, vagina, pubic symphysis, and uterine artery wall. In all tissues from females with conditional and global Rxfp1 gene deletion, collagen staining is stronger and collagen fibers are less organized than in wild-type or heterozygous females. In the uterine artery the arrows show the more intense collagen staining within the vascular smooth muscle cell layer. Arrows with the open arrowhead show endothelial cells. Shown are representative images of at least three animals analyzed at ×10 and ×40 magnifications. Bar = 200 μm (for ×10) and 50 μm (for ×40).
DISCUSSION
The main goal of this study was to identify which cells and tissues are responsible for various relaxin-deficient abnormalities in female reproductive organs during late pregnancy—abnormalities that were previously detected in global gene knockout mutants. We used the Cre/loxP conditional targeting approach to inactivate the Rxfp1 relaxin receptor gene in cells and cell lineages where Tagln-cre transgene, driven by a smooth muscle-specific promoter, was expressed. The females with the conditional knockout of Rxfp1 had some but not all characteristics of the global gene knockout mutants. In conditional mutants, the pubic symphysis was shorter than in littermate controls, but it was not as short as in global knockouts. In all analyzed conditional knockout tissues, there was a clear increase of collagen fiber density similar to what was observed in global knockout mutants. There was a significant decrease in the thickness of the cervical and vaginal luminal epithelium in conditional mutants compared with heterozygotes. The data demonstrate an involvement of differentiated smooth muscle cells or cells, derived from the progenitors expressing smooth muscle markers, in relaxin-induced regulation of extracellular collagen remodeling.
The use of various techniques, such as radioactive ligand binding, in situ analysis, immunohistochemistry, and immunofluorescence, has suggested that a number of different tissues within the female reproductive system expressed RXFP1 [1]. Two previously described LacZ-knockin reporter alleles were used to show that in nonpregnant female mice the Rxfp1 gene is expressed mainly in smooth muscle cells of the uterine myometrium as well as in cells of the lamina propria located directly beneath the uterine and vaginal luminal epithelium [3, 4]. The same pattern was detected in the cervix and vagina of nonpregnant female mice by in situ hybridization [1]. Other data suggested that the expression level of the receptor might vary with the menstrual cycle [16, 17]. The data presented here show that at late pregnancy Rxfp1 is expressed in stromal muscle cells of the reproductive tract as well as in the lamina propria beneath the epithelium. No expression was detected in cervical epithelial cells, but some of the vaginal epithelial cells were positive. We did not detect Rxfp1 expression in the uterine artery, which was previously described using immunodetection in the rat [9]. It is possible that this inconsistency is due to the various techniques used in this and other investigations. It should also be noted that in late pregnant rats the expression of Rxfp1 in vascular smooth muscle cells is significantly reduced [9], and hence the analysis of the gene expression in late pregnant mice might not be sensitive enough. Interestingly, the vascular smooth muscle cells of blood vessels within the cervix were stained, whereas in the vagina they were negative. Not surprisingly, however, strong expression of Rxfp1 was present in the majority of cells in the pubic symphysis.
The Cre transgene used in this study is driven by a smooth muscle-specific Tagln promoter [18]. The analysis of its expression in various tissues of the female reproductive tract confirmed that pattern of expression. Importantly, the Tagln-cre allele did induce recombination and LacZ reporter expression in cells located in the lamina propria, the fibrous ring that surrounds the mucous layer of the luminal epithelium in the cervix or vagina. It was shown previously that these fibroblast-like cells did express smooth muscle markers, especially during late pregnancy, and showed ultrastructural features of myofibroblasts [19]. Thus, in conditional knockouts these cells should have lost Rxfp1 gene expression. Previously, in a series of elegant recombinant tissue experiments with wild-type and knockout tissues, it was shown that relaxin receptor expression in stromal cells is primarily responsible for relaxin-induced proliferative effects in the cervical and vaginal epithelium [8]. The analysis of conditional mutants in our experiments demonstrated that the development of the cervical and vaginal luminal epithelium was indeed affected, suggesting that somatic cells of the lamina propria are at least partially responsible for relaxin effects on epithelial cells in vivo. The reduction of epithelial cell thickness in conditional knockouts corresponded to what was observed in females with complete ablation of Rxfp1. Nevertheless, the cervical length was not affected. The chimeric expression of the Cre transgene, leading to incomplete inactivation of Rxfp1 in all cells, might be responsible for the remaining relaxin signaling. It is also possible that there are RXFP1-expressing cells not targeted in conditional mutants within lower reproductive tract and pubic symphysis. The remaining signaling from such cells might mediate relaxin signaling and contribute to the differences observed in conditional and conventional knockouts. It should also be noted that Rxfp1ko/ko and Rxfp1fl/fl females used in these experiments, although on the same C57BL/6 background and of the same age, were derived from different crosses than the heterozygotes and conditional mutants in our experiments. It is therefore possible that some small differences in genetic background might affect the manifestation of relaxin-deficient phenotypes and observed differences between wild-type Rxfp1fl/fl and heterozygote Rxfp1ko/+ females.
We have established here that the cells of the pubic symphysis strongly express RXFP1 during late pregnancy. These cells undergo a continuous differentiation during pregnancy and in the postpartum period, expressing α-smooth muscle actin and other myofibroblast markers [12] and, as shown here, the Tagln-driven Cre transgene. The pubic symphysis in Rxfp1-deficient females failed to develop into an interpubic ligament and remained as short as in nonpregnant females. In conditional knockouts the pubic ligament was developed; however, its length was significantly shorter than in heterozygous or wild-type females. It has been suggested that a contractile function for pubic symphysis cells with myofibroblast features might contribute to support of the varying mechanical stresses present during pubic bone movement [12]. The mechanism of relaxin action on pubic ligament differentiation is not currently known. However, recent data suggest that nitric oxide, one of the downstream targets of relaxin signaling in smooth muscle cells [20], might be responsible for pubic symphysis relaxation [21].
Despite overall normal anatomical appearance, the histological evaluation of reproductive organs of conditional mutants revealed a common defect. In all tissues analyzed there was a clearly visible increase in collagen fiber density. During pregnancy the cervical and vaginal extensibility and softening are an important adaptation for the passage of embryos at parturition. The histological changes include reduction in stroma thickness, density of collagen fiber bundles, and length of elastin fibers [22, 23]. An increased collagen:total protein ratio was also detected in small renal arteries of relaxin knockout mice [24]. It is not clear how relaxin reduces the density of collagen fiber bundles, but our data suggest that smooth muscle cells might be responsible for relaxin-induced rearrangement of the extracellular matrix in various organs.
Some of the previously described abnormalities, such as the undeveloped nipples and inability to feed the pups by mutant females, were not readily apparent in conditional mutants; they were feeding their pups normally. We did not measure the nipple size in the latter animals, but there was no postnatal lethality as there was with the global knockout due to the inability to nurse. It is possible that other, non-smooth muscle cells have a bigger role in the development of that abnormality. We also did not detect a decrease in embryo weight, as described previously for Rln1 knockouts [10], either in conditional or global Rxfp1 knockouts, despite changes in collagen density for their uterine arteries. In fact, the pups were slightly, but significantly larger in our experiments with Rxfp1 knockouts. Interestingly, in rats the suppression of circulating relaxin throughout the second half of pregnancy by monoclonal antibody or ovariectomy led to an increase in fetal weights compared with controls [5]. Based on our previous data [3, 25–27] it is unlikely that mouse relaxin signals through another receptor, although it is still possible that interaction of RXFP1 with other receptors might account for the differences between Rln1 and Rxfp1 knockouts [28]. Further analysis of these differences in mutant phenotype might be necessary.
The exact target of relaxin in the woman's reproductive tract during pregnancy remains less certain [5]. There is no consensus on the pattern of RXFP1 expression in uterus, vagina, and cervix. Binding of porcine relaxin to the myometrium of the marmoset monkey and human was reported, as was RXFP1 expression in clinical samples of myometrium or leiomyoma and in cultured myometrial cells [1]. However, immunohistochemistry studies of RXFP1, radioactive-labeled relaxin-binding experiments, and RNA analysis suggest receptor expression in glandular and luminal epithelium or endometrial cells [1]. Thus the applicability of data obtained in this report to a human condition and the role of relaxin in pregnancy and parturition require further investigation.
In summary, the Tagln-cre transgene induced the conditional deletion of the relaxin receptor gene in cells of smooth muscle lineage, leading to a reduction of pubic symphysis length and a dramatic increase of collagen fiber density in all reproductive organs of pregnant females. This new conditional allele of the Rxfp1 gene can be used for analysis of relaxin signaling in different cells and tissues in vivo.
ACKNOWLEDGMENT
We thank Dr. Eva Zsigmond and Mr. Aleksey Domozhirov of the University of Texas Health Science Center-Houston, Transgenic Mouse and Stem Cells Core Facility for help with the transgenic mouse production. The embryonic stem cells with targeted Rxfp1 allele were obtained from Helmholtz Zentrum München–Deutsches Forschungszentrum für Gesundheit, EUCOMM Consortium. We thank Ms. Courtney Myhr for editorial comments.
Footnotes
Supported by the National Institute Health grant 5R21HL093605-02.
REFERENCES
- Parry LJ, Vodstrcil LA. Relaxin physiology in the female reproductive tract during pregnancy. Adv Exp Med Biol. 2007;612:34–48. doi: 10.1007/978-0-387-74672-2_4. [DOI] [PubMed] [Google Scholar]
- Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology. 1999;140:445–453. doi: 10.1210/endo.140.1.6404. [DOI] [PubMed] [Google Scholar]
- Kamat AA, Feng S, Bogatcheva NV, Truong A, Bishop CE, Agoulnik AI. Genetic targeting of relaxin and insulin-like factor 3 receptors in mice. Endocrinology. 2004;145:4712–4720. doi: 10.1210/en.2004-0515. [DOI] [PubMed] [Google Scholar]
- Krajnc-Franken MA, van Disseldorp AJ, Koenders JE, Mosselman S, van Duin M, Gossen JA. Impaired nipple development and parturition in LGR7 knockout mice. Mol Cell Biol. 2004;24:687–696. doi: 10.1128/MCB.24.2.687-696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev. 2006;58:7–31. doi: 10.1124/pr.58.1.9. [DOI] [PubMed] [Google Scholar]
- Sherwood OD. An “old hand's” perspective of Relaxin 2004's place along the relaxin trail. Ann N Y Acad Sci. 2005;1041:xxix–xxxv. doi: 10.1196/annals.1282.084. [DOI] [PubMed] [Google Scholar]
- Yao L, Agoulnik AI, Cooke PS, Meling DD, Sherwood OD. Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology. 2008;149:2072–2079. doi: 10.1210/en.2007-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos D, Pintado B, de la Fuente J, Lonergan P, Gutierrez-Adan A. Development and pattern of mRNA relative abundance of bovine embryos cultured in the isolated mouse oviduct in organ culture. Mol Reprod Dev. 2007;74:716–723. doi: 10.1002/mrd.20652. [DOI] [PubMed] [Google Scholar]
- Minami N, Bavister BD, Iritani A. Development of hamster two-cell embryos in the isolated mouse oviduct in organ culture system. Gamete Res. 1988;19:235–240. doi: 10.1002/mrd.1120190303. [DOI] [PubMed] [Google Scholar]
- Dyban AP, Samoshkina NA, Mystkowska EB. The oviduct as a barrier to exogenous thymidine in the early development of the mouse embryo. J Embryol Exp Morphol. 1972;27:163–166. [PubMed] [Google Scholar]
- Faria MJ, Simoes ZL, Lunardi LO, Hartfelder K. Apoptosis process in mouse Leydig cells during postnatal development. Microsc Microanal. 2003;9:68–73. doi: 10.1017/S1431927603030101. [DOI] [PubMed] [Google Scholar]
- Steinetz BG, Beach VL, Kroc RL, Stasilli NR, Nussbaum RE, Nemith PJ, Dun RK. Bioassay of relaxin using a reference standard: a simple and reliable method utilizing direct measurement of interpubic ligament formation in mice. Endocrinology. 1960;67:102–115. doi: 10.1210/endo-67-1-102. [DOI] [PubMed] [Google Scholar]
- Zhao L, Samuel CS, Tregear GW, Beck F, Wintour EM. Collagen studies in late pregnant relaxin null mice. Biol Reprod. 2000;63:697–703. doi: 10.1095/biolreprod63.3.697. [DOI] [PubMed] [Google Scholar]
- Soh YM, Tiwari A, Mahendroo M, Conrad KP, Parry LJ. Relaxin regulates hyaluronan synthesis and aquaporins in the cervix of late pregnant mice. Endocrinology. 2012;153:6054–6064. doi: 10.1210/en.2012-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CP, Parry LJ, Samuel CS, Gehring HM, Lederman FL, Rogers PA, Summers RJ. Increased expression of the relaxin receptor (LGR7) in human endometrium during the secretory phase of the menstrual cycle. J Clin Endocrinol Metab. 2004;89:3477–3485. doi: 10.1210/jc.2003-030798. [DOI] [PubMed] [Google Scholar]
- Kamat AA, Feng S, Agoulnik IU, Kheradmand F, Bogatcheva NV, Coffey D, Sood AK, Agoulnik AI. The role of relaxin in endometrial cancer. Cancer Biol Ther. 2006;5:71–77. doi: 10.4161/cbt.5.1.2289. [DOI] [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami N, Iritani A. Role of the oviduct in the development of the mouse embryo. Mol Reprod Dev. 1993;36:279–281. doi: 10.1002/mrd.1080360231. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93:405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- Deutscher E. Hung-Chang Yao H. Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol. 2007;307:227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sherwood OD. Monoclonal antibodies specific for rat relaxin, X: endogenous relaxin induces changes in the histological characteristics of the rat vagina during the second half of pregnancy. Endocrinology. 1998;139:4726–4734. doi: 10.1210/endo.139.11.6327. [DOI] [PubMed] [Google Scholar]
- Lee AB, Hwang JJ, Haab LM, Fields PA, Sherwood OD. Monoclonal antibodies specific for rat relaxin, VI: passive immunization with monoclonal antibodies throughout the second half of pregnancy disrupts histological changes associated with cervical softening at parturition in rats. Endocrinology. 1992;130:2386–2391. doi: 10.1210/endo.130.4.1547746. [DOI] [PubMed] [Google Scholar]
- Wen Q, Zheng QS, Li XX, Hu ZY, Gao F, Cheng CY, Liu YX. Wt1 dictates the fate of fetal and adult Leydig cells during development in the mouse testis. Am J Physiol Endocrinol Metab. 2014;307:E1131–1143. doi: 10.1152/ajpendo.00425.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Kamat AA, Agoulnik AI. Genetic targeting of relaxin and insl3 signaling in mice. Ann N Y Acad Sci. 2005;1041:82–90. doi: 10.1196/annals.1282.012. [DOI] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Kamat AA, Truong A, Agoulnik AI. Endocrine effects of relaxin overexpression in mice. Endocrinology. 2006;147:407–414. doi: 10.1210/en.2005-0626. [DOI] [PubMed] [Google Scholar]
- Agoulnik AI. Relaxin and related peptides in male reproduction. Adv Exp Med Biol. 2007;612:49–64. doi: 10.1007/978-0-387-74672-2_5. [DOI] [PubMed] [Google Scholar]
- Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD, Samuel CS. Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int. 2014;86:75–85. doi: 10.1038/ki.2013.518. [DOI] [PubMed] [Google Scholar]