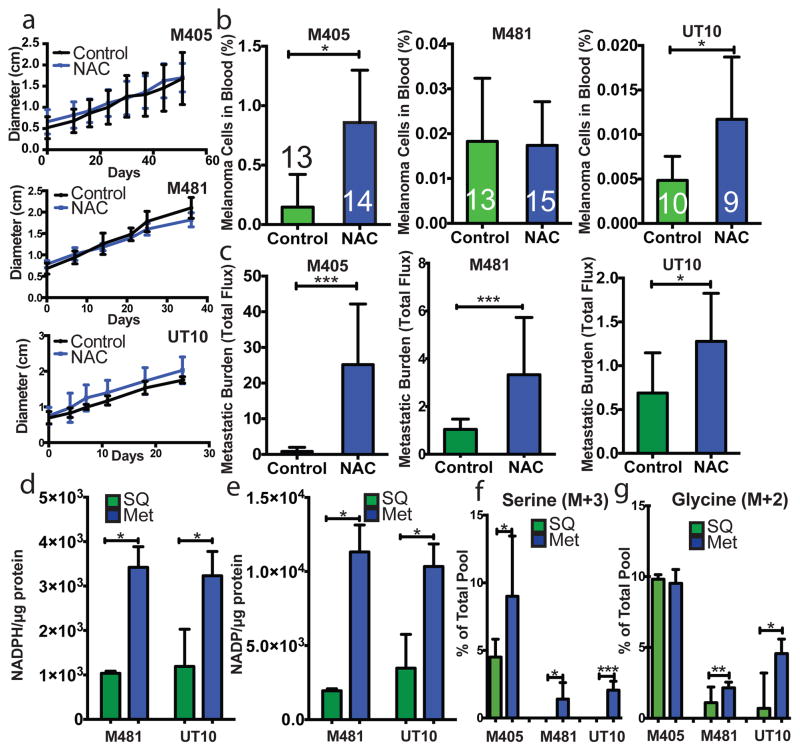
Figure 2. Melanoma cell metastasis, but not subcutaneous tumour growth, is promoted by anti-oxidants in vivo.
a–c) Growth of established subcutaneous tumours in NSG mice treated with either PBS (Control) or N-acetyl-cysteine (NAC) by daily subcutaneous injection. Tumor diameter source data are shown in Supplementary Figure 1. Frequency of circulating melanoma cells in the blood (b) and metastatic disease burden (c) assessed based on total bioluminescence signal from the visceral organs of the same mice. Data in panels a–c represent 8 independent experiments with total replicates/treatment shown in the bars of panel b. Panel a shows a single representative experiment per melanoma due to the difficulty of reflecting tumour growth measurements from independent experiments in the same graph. No statistically significant differences among treatments were observed in subcutaneous tumor growth in any experiment. d and e) Levels of NADPH (d) and NADP (e) in subcutaneous tumours versus metastatic nodules (n=4 mice from 2 independent experiments with M481 and UT10). f and g) In vivo isotope tracing of uniformly 13C-labelled glucose into serine (f), and glycine (g) in subcutaneous tumours versus metastatic nodules from the same mice (n=6 mice in 2 independent experiments for M405; n=3 mice in one experiment for each of M481 and UT10). The fragments for uniformly labeled serine (M+3) and glycine (M+2), which come from labeled glucose via de novo serine synthesis, are shown. All data represent mean±sd. Statistical significance was assessed using two-tailed Student’s t-tests (d–f, h and i), or the Mann-Whitney test (g, due to unequal variance), and repeated-measures two-way analyses of variance (ANOVAs) (a–c; *. p<0.05; **, p<0.005; ***, p< 0.0005, ****, p<0.00005).