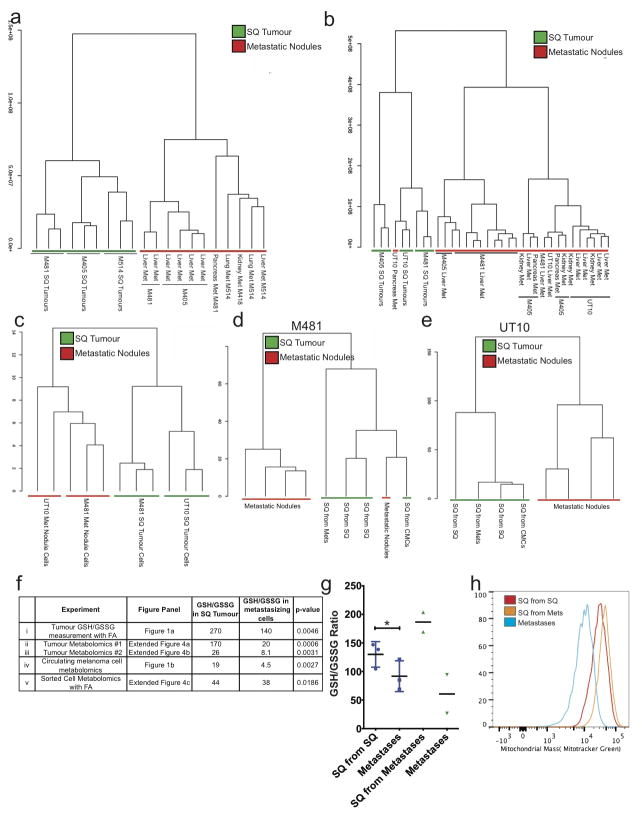
Extended data figure 4. Unsupervised clustering suggests that melanoma cells undergo reversible metabolic changes during metastasis.
Hierarchical clustering from two independent experiments reflecting (a) subcutaneous (SQ) tumours and metastatic nodules from the liver, pancreas, lung, and kidneys of mice transplanted with melanomas M405, M481 and M514 (see Extended data table 1 for data on individual metabolites) and (b) subcutaneous (SQ) tumours and metastatic nodules from the liver, pancreas, and kidneys of mice transplanted with melanomas M405, M481 and UT10 (n=2–3 mice/melanoma in each experiment; see Extended data table 2 for data on individual metabolites). c) Hierarchical clustering of metabolites extracted from flow cytometrically sorted human melanoma cells isolated from subcutaneous tumours or metastatic nodules (UT10, M481, n=3 mice/melanoma in two independent experiments). d and e) Hierarchical clustering of metabolites extracted from subcutaneous tumours and metastatic nodules from mice transplanted subcutaneously with either subcutaneous, circulating or metastatic melanoma cells (n=4 mice for each melanoma in two independent experiments). f) GSH/GSSG ratios from each of the experiments that compared subcutaneous tumours and metastasizing cells. i) Metabolites were extracted in the presence of 0.1% formic acid to inhibit spontaneous oxidation42 in two independent experiments comparing subcutaneous and metastatic tumours from mice with three different melanomas in each experiment (M405, M481, and UT10). ii and iii) GSH/GSSG ratios from the experiments shown in panels a and b, respectively. iv) GSH/GSSG ratios in melanoma cells isolated by flow cytometry from subcutaneous tumours and the blood (CMCs) of mice bearing M405 and M481. v) Metabolites were extracted in the presence of 0.1% formic acid in two independent experiments in which melanoma cells were isolated by flow cytometry from subcutaneous and metastatic tumours (M405 and M481). While the GSH/GSSG ratio was always significantly higher in melanoma cells from subcutaneous tumours as compared to circulating cells or metastatic nodules the ratio varied among experiments as a result of technical differences in cell isolation and metabolite extraction as well as differences in MS sensitivity to GSH and GSSG. g) GSH/GSSG ratios in subcutaneous tumours that arose from the transplantation of melanoma cells obtained from subcutaneous tumours or metastatic nodules, as well as the metastatic nodules from the same mice (M405; n=2 to 3 mice per treatment in one experiment). These data suggest that the decline in GSH/GSSG ratio in metastasizing melanoma cells is reversible upon subcutaneous transplantation. h) Histogram showing mitochondrial mass in subcutaneous tumour cells that arose from the transplantation of subcutaneous cells (SQ from SQ), subcutaneous tumour cells that arose from the transplantation of metastatic cells (SQ from Mets), and metastatic cells (Metastases). These histograms reflect the data shown in Figure 1g. All error bars represent standard deviation. Statistical significance was assessed using, two-tailed Student’s t-tests (f and g) (*, p<0.05)