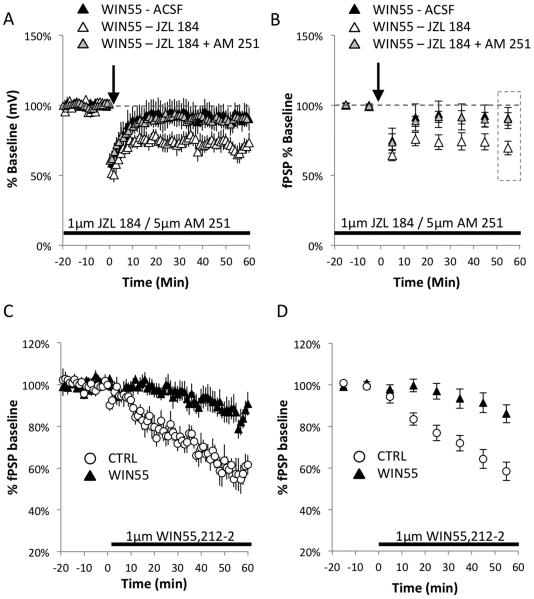
Figure 3.
Inhibition of 2-AG hydrolysis ameliorates the eCB-LTD deficiency at L2/3→L5 synapses.
(A) The WIN55-treated group showed no induction of LTD at the L2/3→L5 synapse in the mPFC. However, 10-min stimulation of slices from WIN55-treated mice at 10 Hz in the presence of 1 µM JZL 184 rescued the deficit (Wilcoxon test: WIN55-treated+JZL 184: 69.46% ± 4.90%, Z = −2.366, n = 7 p = 0.018). In addition, an attempt to rescue impaired eCB-LTD in the WIN55-treated mice with 1 µM JZL 184 failed in the presence of selective blocker of CB1R function AM 251 (5 µM). (B) The binned averages of 10 min time segments (calculated based on data in (A) show distinct differences under all experimental conditions. Data were analyzed from the binned average time point in the rectangle marked during the last 10 min of recording. While WIN55-treated slices showed no induction of eCB-LTD, JZL 184 application to WIN55 slices enhanced LTD to control levels (Figure 1E), which failed in the presence of CB1R blocker AM 251 (C) CB1R function is reduced in WIN55- treated mice. The WIN55-treated group shows decreased functionality of CB1R at the L2/3→L5 synapse in the mPFC compared to control mice. After 20 min of baseline recording, continuous application of 1 µM WIN55,212-2, a CB1R agonist, caused a reduction of fPSP amplitude in the control group; this reduction was markedly dampened in the WIN55-treated mice. (D) Analysis of binned averages showed a markedly dampened effect in the WIN55-treated mice.