Abstract
Background
Ciprofloxacin is a commonly used antibiotic for urinary tract infection that interacts with bacterial topoisomerases leading to oxidative radicals generation and bacterial cell death. Phosphodiesterase inhibitors (PDEis), on the other hand, are commonly used drugs for the management of erectile dysfunction. The group includes agents such as sildenafil, vardenafil, and tadalafil.
Objectives
We investigated whether PDEi could interfere with the antibacterial activity of ciprofloxacin.
Methods
PDEis were tested in several reference bacteria, including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermidis, Acinetobacter baumannii, Proteus mirabilis, and Klebsiella pneumoniae utilizing a standard disc diffusion method and measuring both zones of inhibition and MIC.
Results
Results from both assays indicated that ciprofloxacin demonstrates potent activity against the tested reference bacteria. Additionally, when bacteria were treated with a combination of ciprofloxacin and sildenafil, tadalafil, or vardenafil, the zones of the combination inhibition were significantly reduced, whereas the MIC values were significantly greater than those of ciprofloxacin alone for all tested bacterial strains. In an attempt to examine the mechanism by which PDEis interfere with the action of ciprofloxacin, we utilized the in vitro E coli DNA gyrase cleavage assay. The results showed that PDEi drugs had no effect on ciprofloxacin’s inhibition of E coli gyrase activity.
Conclusions
Pretreatment of various reference bacterial cells with PDEis largely inhibited the antibacterial activity of ciprofloxacin.
Key words: antimicrobial susceptibility, ciprofloxacin, MIC, PDEi, sildenafil, tadalafil, vardenafil
Introduction
Phosphodiesterase inhibitors (PDEis) are a widely used group of oral therapy for erectile dysfunction. This group is selective for cyclic guanosine monophosphate-specific phosphodiesterase (PDE) type 5 present in corpora cavernosa.1 The group has 3 major members: sildenafil, vardenafil, and tadalafil.2 These agents differ in their degree of selectivity in inhibiting PDE isoenzymes, in their pharmacokinetic profiles, in their drug-food interactions, and in their adverse effects.[1], [3] These agents have been shown to possess antioxidative or oxidative stress-protective properties.[4], [5]
Ciprofloxacin is a fluoroquinolone antibiotic that possesses strong activity against gram-negative bacteria. Ciprofloxacin is commonly used for the treatment of a number of infections such as acute uncomplicated cystitis, urinary tract infections, acute sinusitis, and chronic bacterial prostatitis.6 The mechanism of antibacterial action of quinolone, including ciprofloxacin, involves interfering with replication and transcription of DNA via inhibiting bacterial DNA gyrase/topoisomerase II and DNA topoisomerase IV, and further preventing DNA of bacteria from unwinding and duplicating.7 Thus, complexes of quinolone-enzyme-DNA are formed, leading to the production of cellular poisons and cell death.8
Microbiologic studies of various bacteria ascertain the presence of the guanosine monophosphate-PDE system in bacteria,9 which could represent a possible pharmacologic target for sildenafil and similar agents in bacteria.10 Moreover, a previous study11 showed that coadministration of ciprofloxacin and clarithromycin significantly increased sildenafil bioavailability in human beings. This could point to a possible interaction with antibiotic agents that are commonly administrated concomitantly with these agents. We evaluated, for the first time, the possible interaction among members of the PDEi group and ciprofloxacin. The results of our study could be of clinical significance due to the common use of PDEis, especially, sildenafil, when antibiotics are used for treatment of urinary tract infection.
Materials and Methods
Chemicals
Ciprofloxacin used in this study was donated by Al-Hikma Pharmaceuticals (Amman, Jordan). Sildenafil was obtained from Sigma-Aldrich Corporation (St Louis, Missouri). Vardenafil and tadalafil were obtained from Orchid Chemical Supplies Ltd (Hangzhou, China). All drugs were used as raw material.
Microbial culture and growth conditions
Antibacterial activity of ciprofloxacin and/or PDEi combinations were evaluated against different reference bacteria, including Escherichia coli ATTC 35218, Staphylococcus aureus ATTC29213, Pseudomonas aeruginosa ATTC 9027, Staphylococcus epidermidis ATTC 12228, Acinetobacter baumannii ATTC 17978, Proteus mirabilis ATTC 12459, and Klebsiella pneumoniae ATTC 13883. The organisms were stored at –70°C in trypticase-soy broth and 20% glycerol (BBL Microbiology Systems, Cockeysville, Maryland). When ready for batch susceptibility testing, samples were thawed. MICs were determined in accordance with the Clinical and Laboratory Standards Institute.12
Antimicrobial susceptibility test
Antibiotic solutions were prepared on the day of use according to the manufacturer’s recommendations. A wide range of ciprofloxacin concentrations were tested against different organisms. Serial 2-fold dilutions were added to molten BBL Muller-Hinton Gold II agar (BBL Microbiology Systems). After slight cooling and drying of the plates, a steers replicator was used to place aliquots containing approximately 5 × 104 CFU per drop for 4 test strains. The plates were incubated at 37°C and read 24 hours later. In experiments where 0.1 µg/mL ciprofloxacin was combined with PDEi, PDEis were added to the media at a final concentration of 100 µM. Results (ie, the mean of 3 independent experiments) were recorded by measuring the zones of growth inhibition surrounding the antibiotic-containing discs. The breakpoints indicated in the tables of the Clinical and Laboratory Standards Institute guidelines12 were used to determine susceptibility and resistance.
Determination of MIC
The MICs were determined by serial dilution method as described previously.13 Briefly, drugs were serially diluted and added to 96-well plates that were prepared by
dispensing into each well 100 µL of an appropriate medium (BBL Muller-Hinton Gold II agar; BBL Microbiology Systems) and 20 µL inoculum (containing about 5 × 104 CFU). After an 18-hour incubation period at 37°C, plates were read. MIC is defined as the lowest concentration at which no growth, a faint haze, or fewer than 3 discrete colonies was detected. Plates were read in duplicate and the highest MIC value was recorded.
E coli DNA gyrase cleavage assay
The effect of PDEis on antigyrase activity of ciprofloxacin was examined using the E coli DNA gyrase cleavage assay as described by the manufacturer (Inspirals, Norwich, United Kingdom). In brief, DNA gyrase was incubated with 0.5 µg supercoiled pBR322 in a reaction volume at 37°C for 1 hour in the presence of 0.1 µg/mL ciprofloxacin and/or different PDEis (100 µM). SDS and proteinase K (0.2% and 0.1 µg/mL final concentrations, respectively) were added before a further incubation at 37°C for 30 minutes. About 10 µL reaction mixture was electrophoresized using 1% agarose and bands were visualized using ethidium bromide.
Statistical analysis
Analysis was performed using GraphPad Prism software (version 4.0, GraphPad Software, La Jolla, California). One-way ANOVA followed by Tukey’s posttest were used to determine if there was any statistically significant difference. P values < 0.05 were considered significant.
Results
We investigated the possible attenuating effect of a PDEi on the antibacterial activity of ciprofloxacin against various species of reference bacteria, namely, E coli, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermidis, A baumannii, Proteus mirabilis, and K pneumoniae. Inhibition zones suggested in the Clinical and Laboratory Standards Institute guidelines were considered representative of bacterial susceptibility to the compounds.12 Table I shows that ciprofloxacin possessed significant antibacterial activity against the reference bacteria that were tested, except for A baumannii and K pneumonia, which showed a zone of inhibition in the intermediate and resistant ranges. When reference strains were treated with a combination of ciprofloxacin with sildenafil, tadalafil, or vardenafil, the zones of inhibition of the combination were significantly lower than those of ciprofloxacin alone for all tested bacterial strains (Table I).
Table I.
Comparison among the zones of inhibition (mm) of ciprofloxacin alone and ciprofloxacin in the presence of sildenafil, tadalafil, or vardenafil against standard bacterial strains
| Standard bacterial strains | Zones of inhibition (mm)* |
|||
|---|---|---|---|---|
| Ciprofloxacin | Ciprofloxacin + sildenafil | Ciprofloxacin + tadalafil | Ciprofloxacin + vardenafil | |
| Escherichia coli | 26.7 (0.6) | 11.3 (1.5) | 11.0 (1.0) | 11. 7 (0.6) |
| Staphylococcus aureus | 21 (1.0) | 9. 7 (1.2) | 9. 7 (0.6) | 9.3 (1.5) |
| Pseudomonas aeruginosa | 23.3 (0.6) | 11 (1.0) | 10. 7 (0.6) | 7.0 (2.0) |
| Staphylococcus epidermidis | 21. 7 (0.6) | 10.3 (1.2) | 10.3 (0.6) | 11.3 (0.6) |
| Acinetobacter baumannii | 17. 7 (0.6) | 8.3 (0.6) | 7. 7 (0.6) | 8.3 (0.6) |
| Proteus mirabilis | 18. 7 (0.6) | 8. 7 (0.6) | 8. 7 (0.6) | 7. 7 (0.6) |
| Klebsiella pneumoniae | 12.0 (1.0) | 4. 7 (0.6) | 6. 7 (0.6) | 5. 7 (0.6) |
The zones of inhibition values for ciprofloxacin alone were significantly (P < 0.05) lower than those of combination of ciprofloxacin with sildenafil, tadalafil, or vardenafil for all tested bacterial strains. Results are presented as mean (SD) of 3 independent experiments.
Next, the MICs of ciprofloxacin alone and the combination of ciprofloxacin with sildenafil, tadalafil, or vardenafil were measured for all tested strains. As shown in Table II, pretreatment of various reference bacteria cells with a PDEi largely inhibited the antibacterial activity of ciprofloxacin. This is indicated by significantly higher MIC values (Table II) for the combination of any of the PDEis (sildenafil, vardenafil, or tadalafil) and ciprofloxacin compared with ciprofloxacin alone.
Table II.
Comparison between the MICs (µg/mL) of ciprofloxacin alone and ciprofloxacin in the presence of sildenafil, tadalafil, or vardenafil against standard bacterial strains
| Standard bacterial strains | MIC (µg/mL)* |
|||
|---|---|---|---|---|
| Ciprofloxacin | Ciprofloxacin + sildenafil | Ciprofloxacin + tadalafil | Ciprofloxacin + vardenafil | |
| Escherichia coli | 0.02 (0.01) | 1300 (100) | 1700 (100) | 1800 (100) |
| Staphylococcus aureus | 0.07 (0.05) | 1167 (58) | 1600 (100) | 1833 (58) |
| Pseudomonas aeruginosa | 0.07 (0.05) | 1267 (58) | 1700 (100) | 1867 (58) |
| Staphylococcus epidermidis | 0.14 (0.09) | 1100 (100) | 1500 (100) | 1700 (100) |
| Acinetobacter baumannii | 0.21 (0.07) | 1400 (100) | 1700 (100) | 1767 (58) |
| Proteus mirabilis | 0.17 (0.07) | 1600 (100) | 1900 (100) | 1933 (58) |
| Klebsiella pneumoniae | 0.14 (0.09) | 933 (57) | 1600 (100) | 1733 (58) |
We investigated whether PDEi could interfere with the antibacterial activity of ciprofloxacin.
Ciprofloxacin antibacterial action is inhibited when combined with PDEi
This observation is of significance, as ciprofloxacin is a commonly used antibiotic
The MIC values for ciprofloxacin alone were significantly (P < 0.05) lower than those of combination of ciprofloxacin with sildenafil, tadalafil, or vardenafil for all tested bacterial strains. Results are presented as mean (SD) of 3 independent experiments.
To examine the mechanism by which PDEis interfere with the action of ciprofloxacin, the in vitro E coli DNA gyrase cleavage assay was used. The results showed that ciprofloxacin significantly inhibited E coli gyrase activity. However, treatment with PDEi drugs had no effect on ciprofloxacin-induced inhibition of E coli gyrase activity (Figure 1). Moreover, PDEi drugs alone did not affect E coli gyrase activity (data not shown).
Figure 1.
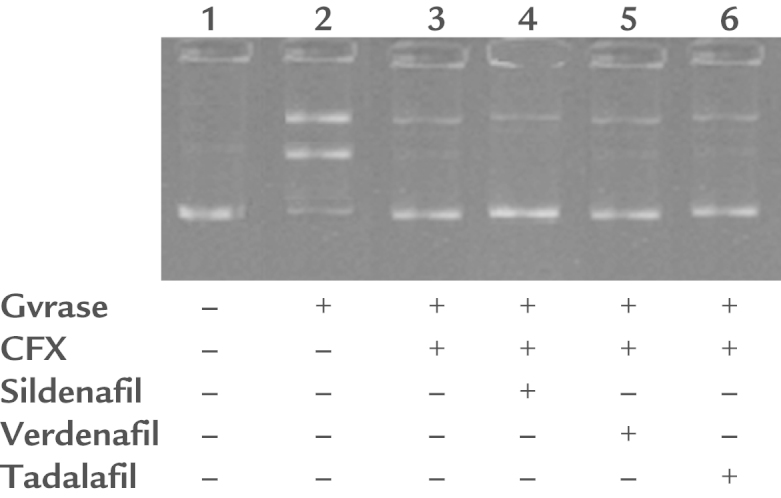
The Escherichia coli DNA gyrase cleavage assay. DNA gyrase was incubated with supercoiled pBR322 in the presence of 0.1 µg/ML ciprofloxacin (CFX) and/or 1 of the phosphodiesterase inhibitors (100 µM). Bands were separated using 1% agarose and visualized using ethidium bromide. Phosphodiesterase inhibitors (100 µM) did not affect antigyrase activity of 0.1 µg/mL CFX.
Discussion
Our study showed, for the first time, the inhibition of the antibacterial activity of ciprofloxacin when bacteria are pretreated with any of the PDEis. These results were generated using wide panel of standard bacterial strains and they could be of importance when ciprofloxacin is used on top of PDEis to treat bacterial infections in older men.
Our results show the efficacy of ciprofloxacin on variety of bacterial strains, including E coli, S aureus, Pseudomonas aeruginosa, S epidermidis, and Proteus mirabilis. In accordance, previous studies have shown the susceptibility of these bacterial strains to ciprofloxacin.[13], [14] We and others have previously demonstrated the crucial role of reactive oxygen species in the antibacterial action of ciprofloxacin on bacterial species, including Pseudomonas aeruginosa, E coli, and S aureus.[13], [15], [16], [17] On the other hand, common scavengers of reactive oxygen species, including vitamin C and vitamin Ε, were shown to attenuate the antibacterial activity of ciprofloxacin.13 Additionally, it was shown that ciprofloxacin induces reactive oxygen species production when it works against bacterial strains such as E coli, Enterococcus faecalis, and S aureus.16 Furthermore, elevated reactive oxygen species levels were shown in ciprofloxacin-sensitive microorganisms.17 For example, increased levels of intracellular superoxide were reported in ciprofloxacin-sensitive microorganisms compared with the resistant ones. It was also shown that ascorbic acid or glutathione application attenuated the antibacterial activity of ciprofloxacin against Escherichia coli, which was dependent on superoxide anions and hydrogen peroxide scavenging.18
Our present results indicate that combining ciprofloxacin with a PDEi results in inhibition of the antibacterial activity of ciprofloxacin against a panel of reference bacterial strains. To our knowledge, this is the first report of such effect or drug-drug interaction. This could point out that concurrent use of ciprofloxacin with any of the PDEis we tested might oppose the antibacterial activity of this antibiotic. Therefore, PDEi use might need to be closely monitored in patients who are receiving ciprofloxacin.
The mechanism for this interactive effect of ciprofloxacin and PDEis is unknown. Quinolones exert their bactericidal actions through the inhibition of DNA gyrase, bacterial type II topoisomorase.[19], [20] Yet multiple other effects were related to quinolones, such as inhibiting the growth of other types of cells[21], [22], [23], [24], [25] via interference with cell cycles, reducing cell size,25 inhibiting de novo synthesis of pyrimidine,25 and interfering with mitochondrial enzymes that are involved in energy metabolism21 and oxidative stress.[18], [26]
The PDEis are known to inhibit PDE isoenzymes.1 However, these agents were shown to possess other effects, such as being antioxidative or oxidative stress protective,[4], [5] being immunomodulatory, having anti-inflammatory properties,27 and altering energy metabolism and mitochondrial biogenesis.28 Given the importance of reactive oxygen species, energy metabolism, and mitochondrial functions for the antibacterial action of ciprofloxacin,[13], [15], [16], [17] it is possible that these mechanisms play a role in the observed inhibition of the antibacterial activity of ciprofloxacin by PDEi family members. The results showed an absence of effect for PDEi drugs on the gyrase inhibitory action of ciprofloxacin. Thus, it is unlikely that PDEi interacts directly with ciprofloxacin and prevents its antigyrase activity. Future studies are needed to indicate the exact mechanism by which PDEis interfere with the action of ciprofloxacin.
The concentration of PDEis used in this study was generally lower than that in human plasma as judged from the pharmacokinetic profile of each PDEi.[29], [30], [31] However, taking into consideration the fraction of each PDEi that is eliminated in the urine—about 15% for sildenafil29—the used concentration in our study becomes reasonable. Our study shows the concept of the possible drug-drug interaction between PDEi family members and ciprofloxacin. Future work should focus on a range of relevant concentrations to further characterize the effect observed in our study.
Conclusions
The antibacterial action of ciprofloxacin is inhibited when combined with a PDEi, including sildenafil, tadalafil, or vardenafil. This observation is significant because ciprofloxacin is a commonly used antibiotic with huge therapeutic value.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article
Acknowledgment
The authors thank Jordan University of Science and Technology, Irbid, Jordan, for providing financial support (grant No. 117-2013). M.M. Masadeh designed the study, carried out the antimicrobial susceptibility and MIC determination experiments, and collected data. He also participated in data interpretation, figure creation and manuscript writing. K.H.Alzoubi participated in literature review, study design, data collection, figure creation, data interpretation and drafted the manuscript. O.F.Khabour participated in study design, carried out the DNA Gyrase experiments, collected data and participated in data interpretation. He also helped in drafting the manuscript. S.I.Al-Azzam carried out literature review, participated in study design, data collection and in manuscript drafting.
References
- 1.Lombardi G., Nelli F., Celso M., Mencarini M., Del Popolo G. Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med. 2012;9:970–985. doi: 10.1111/j.1743-6109.2011.02615.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith W.B., 2nd, McCaslin I.R., Gokce A., Mandava S.H., Trost L., Hellstrom W.J. PDE5 inhibitors: considerations for preference and long-term adherence. Int J Clin Pract. 2013;67:768–780. doi: 10.1111/ijcp.12074. [DOI] [PubMed] [Google Scholar]
- 3.Patel D.N., Li L., Kee C.L., Ge X., Low M.Y., Koh H.L. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges. J Pharm Biomed Anal. 2014;87:176–190. doi: 10.1016/j.jpba.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Guzman D.C., Olguin H.J., Brizuela N.O., Garcia E.H., Mejia G.B., Jacobo A.J., Abarca L.S., Betancourt E.T. Effect of prostaglandin E1 (PGE1) and sildenafil on serotonin metabolism and some oxidative damage markers in rat prostate gland and brain. Andrologia. 2011;43:266–272. doi: 10.1111/j.1439-0272.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 5.Puerta E., Barros-Minones L., Hervias I., Gomez-Rodriguez V., Orejana L., Pizarro N., de la Torre R., Jordan J., Aguirre N. Long-lasting neuroprotective effect of sildenafil against 3,4-methylenedioxymethamphetamine- induced 5-hydroxytryptamine deficits in the rat brain. J Neurosci Res. 2012;90:518–528. doi: 10.1002/jnr.22759. [DOI] [PubMed] [Google Scholar]
- 6.Castro W., Navarro M., Biot C. Medicinal potential of ciprofloxacin and its derivatives. Future Med Chem. 2013;5:81–96. doi: 10.4155/fmc.12.181. [DOI] [PubMed] [Google Scholar]
- 7.Nam Y.S., Cho S.Y., Yang H.Y., Park K.S., Jang J.H., Kim Y.T., Jeong J.W., Suh J.T., Lee H.J. Investigation of mutation distribution in DNA gyrase and topoisomerase IV genes in ciprofloxacin-non-susceptible Enterobacteriaceae isolated from blood cultures in a tertiary care university hospital in South Korea, 2005-2010. Int J Antimicrob Agents. 2013;41:126–129. doi: 10.1016/j.ijantimicag.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Strahilevitz J., Jacoby G.A., Hooper D.C., Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin N., Baehr W. Expression of mouse rod photoreceptor cGMP phosphodiesterase gamma subunit in bacteria. FEBS Lett. 1993;321:6–10. doi: 10.1016/0014-5793(93)80609-x. [DOI] [PubMed] [Google Scholar]
- 10.Li N., Xi Y., Tinsley H.N., Gurpinar E., Gary B.D., Zhu B., Li Y., Chen X., Keeton A.B., Abadi A.H. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/beta-catenin signaling. Mol Cancer Ther. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedaya M.A., El-Afify D.R., El-Maghraby G.M. The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers. Biopharm Drug Dispos. 2006;27:103–110. doi: 10.1002/bdd.488. [DOI] [PubMed] [Google Scholar]
- 12.CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 22end Informational supplement, Villanova, NCCLS, M100-S22. 2012.
- 13.Masadeh M.M., Mhaidat N.M., Alzoubi K.H., Al-Azzam S.I., Shaweesh A.I. Ciprofloxacin-induced antibacterial activity is reversed by vitamin E and vitamin C. Curr Microbiol. 2012;64:457–462. doi: 10.1007/s00284-012-0094-7. [DOI] [PubMed] [Google Scholar]
- 14.Campoli-Richards D.M., Monk J.P., Price A., Benfield P., Todd P.A., Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35:373–447. doi: 10.2165/00003495-198835040-00003. [DOI] [PubMed] [Google Scholar]
- 15.Umezawa N., Arakane K., Ryu A., Mashiko S., Hirobe M., Nagano T. Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Arch Biochem Biophys. 1997;342:275–281. doi: 10.1006/abbi.1997.0124. [DOI] [PubMed] [Google Scholar]
- 16.Albesa I., Becerra M.C., Battan P.C., Paez P.L. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun. 2004;317:605–609. doi: 10.1016/j.bbrc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 17.Becerra M.C., Albesa I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem Biophys Res Commun. 2002;297:1003–1007. doi: 10.1016/s0006-291x(02)02331-8. [DOI] [PubMed] [Google Scholar]
- 18.Goswami M., Mangoli S.H., Jawali N. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob Agents Chemother. 2006;50:949–954. doi: 10.1128/AAC.50.3.949-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gootz T.D., Barrett J.F., Sutcliffe J.A. Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob Agents Chemother. 1990;34:8–12. doi: 10.1128/aac.34.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence J.W., Darkin-Rattray S., Xie F., Neims A.H., Rowe T.C. 4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells. J Cell Biochem. 1993;51:165–174. doi: 10.1002/jcb.240510208. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence J.W., Claire D.C., Weissig V., Rowe T.C. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol Pharmacol. 1996;50:1178–1188. [PubMed] [Google Scholar]
- 23.Nordmann P., Pechinot A., Kazmierczak A. Cytotoxicity and uptake of pefloxacin, ciprofloxacin, and ofloxacin in primary cultures of rat hepatocytes. J Antimicrob Chemother. 1989;24:355–363. doi: 10.1093/jac/24.3.355. [DOI] [PubMed] [Google Scholar]
- 24.Oomori Y., Yasue T., Aoyama H., Hirai K., Suzue S., Yokota T. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J Antimicrob Chemother. 1988;22 Suppl D:91–97. doi: 10.1093/jac/22.supplement_d.91. [DOI] [PubMed] [Google Scholar]
- 25.Forsgren A., Bredberg A., Pardee A.B., Schlossman S.F., Tedder T.F. Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth. Antimicrob Agents Chemother. 1987;31:774–779. doi: 10.1128/aac.31.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurbay A., Hincal F. Ciprofloxacin-induced glutathione redox status alterations in rat tissues. Drug Chem Toxicol. 2004;27:233–242. doi: 10.1081/dct-120037504. [DOI] [PubMed] [Google Scholar]
- 27.Lubamba B., Huaux F., Lebacq J., Marbaix E., Dhooghe B., Panin N., Wallemacq P., Leal T. Immunomodulatory activity of vardenafil on induced lung inflammation in cystic fibrosis mice. J Cyst Fibros. 2012;11:266–273. doi: 10.1016/j.jcf.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 28.De Toni L., Strapazzon G., Gianesello L., Caretta N., Pilon C., Bruttocao A., Foresta C. Effects of type 5-phosphodiesterase inhibition on energy metabolism and mitochondrial biogenesis in human adipose tissue ex vivo. J Endocrinol Invest. 2011;34:738–741. doi: 10.1007/BF03346724. [DOI] [PubMed] [Google Scholar]
- 29.Muirhead G.J., Rance D.J., Walker D.K., Wastall P. Comparative human pharmacokinetics and metabolism of single-dose oral and intravenous sildenafil. Br J Clin Pharmacol. 2002;53 Suppl 1:13S–20S. doi: 10.1046/j.0306-5251.2001.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forgue S.T., Patterson B.E., Bedding A.W., Payne C.D., Phillips D.L., Wrishko R.E., Mitchell M.I. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–288. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischoff E. Vardenafil preclinical trial data: potency, pharmacodynamics, pharmacokinetics, and adverse events. Int J Impot Res. 2004;16(Suppl 1):S34–S37. doi: 10.1038/sj.ijir.3901213. [DOI] [PubMed] [Google Scholar]


