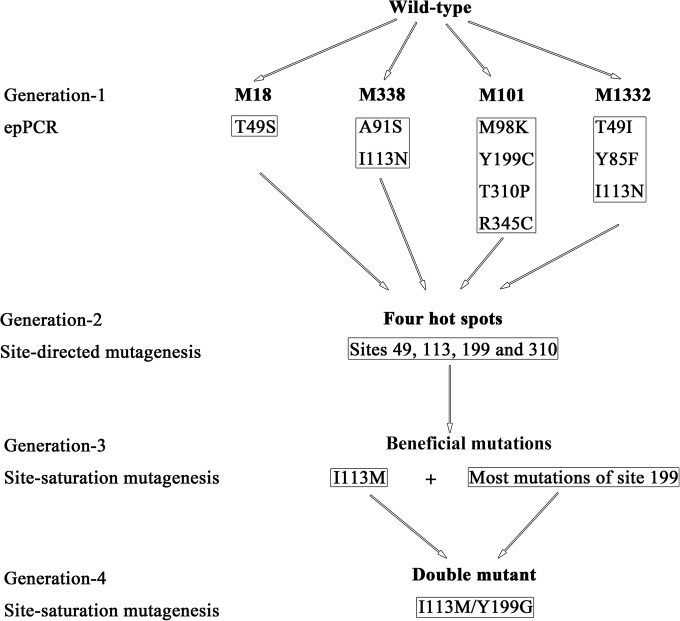
FIG 1.
Scheme of the protein engineering of nitrilase BCJ2315. Initially, random mutagenesis by error-prone PCR (epPCR) yielded four BCJ2315 mutants with enhanced enantioselectivity but decreased activity containing 10 different amino acid substitutions. Site-directed mutagenesis was conducted, and four hot spots (sites 49, 113, 199, and 310) responsible for enantioselectivity and activity of BCJ2315 were characterized. Subsequently, two rounds of site saturation mutagenesis were performed, first at each of the four hot spots and subsequently at position 199 for combination with the selected beneficial mutation I113M, and a I113M/Y199G double mutant was generated that showed pronounced enhancements in enantioselectivity (99.1% ee) and relative activity (360%) compared to those of the WT.