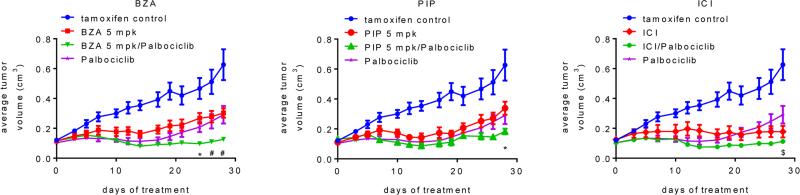
Figure 4. Palbociclib increases the efficacy with which SERDs or SERM/SERD hybrids inhibit the growth of tamoxifen-resistant breast tumor xenografts.
TamR tumors were implanted into tamoxifen-treated mice. When Tam-stimulated tumors attained ~0.1cm3 tumor volume, animals were randomized (7-9 mice per group) to receive continued tamoxifen treatment as well as vehicle or SSH/SERD (BZA, 5 or 10 mg/kg/day s.c.; PIP, 5 or 10 mg/kg/day s.c.; or ICI, 5 mg/mouse 1× weekly i.m.), and also vehicle or palbociclib (100 mg/kg/day, p.o.). Tumor growth for each group (separated by SERD treatment for legibility) is presented as average tumor volume +/− SEM per study arm at each day of treatment, with the initial day of treatment at randomization considered to be day 0. Tam control and palbociclib only treatments presented on each graph are identical. Tumor growth for animals treated with 5 mg/kg BZA or PIP are shown above, while measurements for animals treated with 10 mg/kg BZA or PIP are depicted in Suppl. Figure 4C. By day 14, responses to all treatments were significant as compared to the tam treatment only control (p < 0.01). Significant differences (p < 0.05) between combination treatments and SERD only (*), palbociclib only ($), or both single treatments (#) are indicated at appropriate time points.