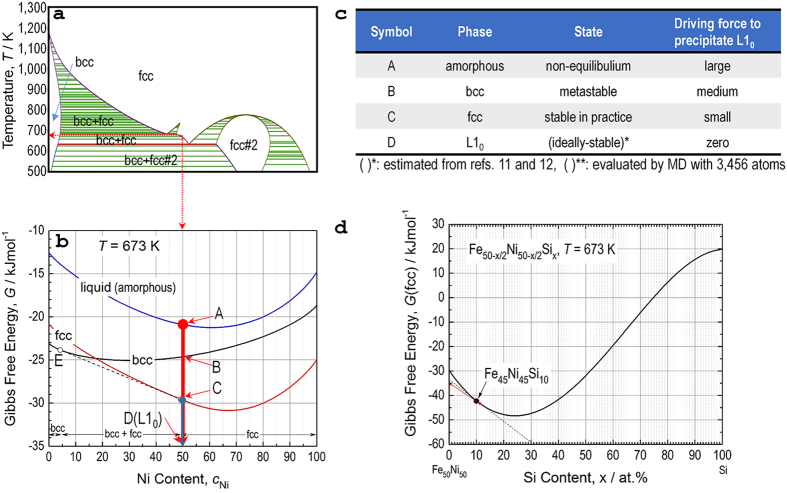
Figure 4. Calculated results and theoretical consideration for forming L10 from the amorphous and crystalline phases.
(a) FeNi phase diagram calculated with SSOL5 database. (b) Gibbs free energy curves at T = 673 K for liquid, bcc, and fcc phases calculated with the SSOL5 database as functions of the Ni fraction together with G = –35 kJmol–1 for the L10 phase estimated from previous studies. (c) Characteristics of the phases of interest, including the driving force to precipitate L10 at T = 673 K. (d) Changes in Gibbs free energy (G) of fcc phase as a function of Si content in a hypothetical Fe50Ni50-Fe50−x/2Ni50−x/2Six system where G = –35 kJmol−1 was considered for L10 Fe50Ni50 phase. Based on lever rule (marked by red arrow), the volume fraction of the L10 phase is evaluated to be ~13% in Fe42Ni41.3Si8B4P4Cu0.7 alloy under an assumption that L10 FeNi phase precipitates from the fcc Fe45Ni45Si10, which is an equilibrium phase at T = 673 K in the alloy.