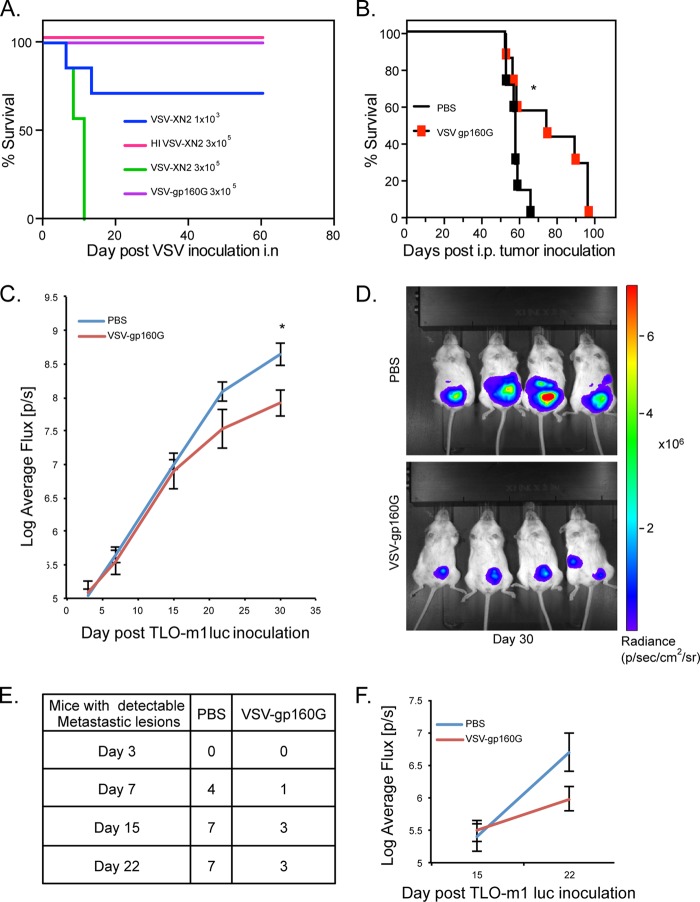
FIG 6.
VSV-gp160G is nonpathogenic within immunodeficient NSG mice and mediates tumor burden relief in ATL bearing hosts. (A) Survival curve of NSG mice (n = 7) inoculated with various doses of VSV-gp160G, VSV-XN2, or heat-inactivated VSV-XN2. VSV vectors were prepared in PBS and delivered intranasally to anesthetized NSG mice. (B) Survival curve of NSG (n = 7) mice were inoculated with 4 × 105 TLO-m1-luc i.p. on day 0 and treated with two injections of VSV-gp160G with 2 × 106 PFU on day 3 and 1 × 107 PFU on day18 (*, P = 0.039, log-rank test). (C) Control or VSV-gp160G-treated mice from panel B were anesthetized and injected with a luciferin substrate i.p., and the uciferase activity was detected on days 3, 7, 15, and 22 using IVIS. The average flux (p/s) emitted is an indicator of tumor burden and was quantified using Living Image software (*, P = 0.014; Student t test two tailed, equal variance). (D) Representative images acquired on day 30 of the experiment when statistical significance was achieved. (E) Numbers of NSG mice inoculated with TLO-m1-luc that developed luciferase activity in areas other than the primary injection site, indicative of significant metastasis. (F) The luciferase activity detected in the metastatic lesions for each group was quantified using Living Image software with control mice (n = 7) and VSV-gp160G-treated mice (n = 3).