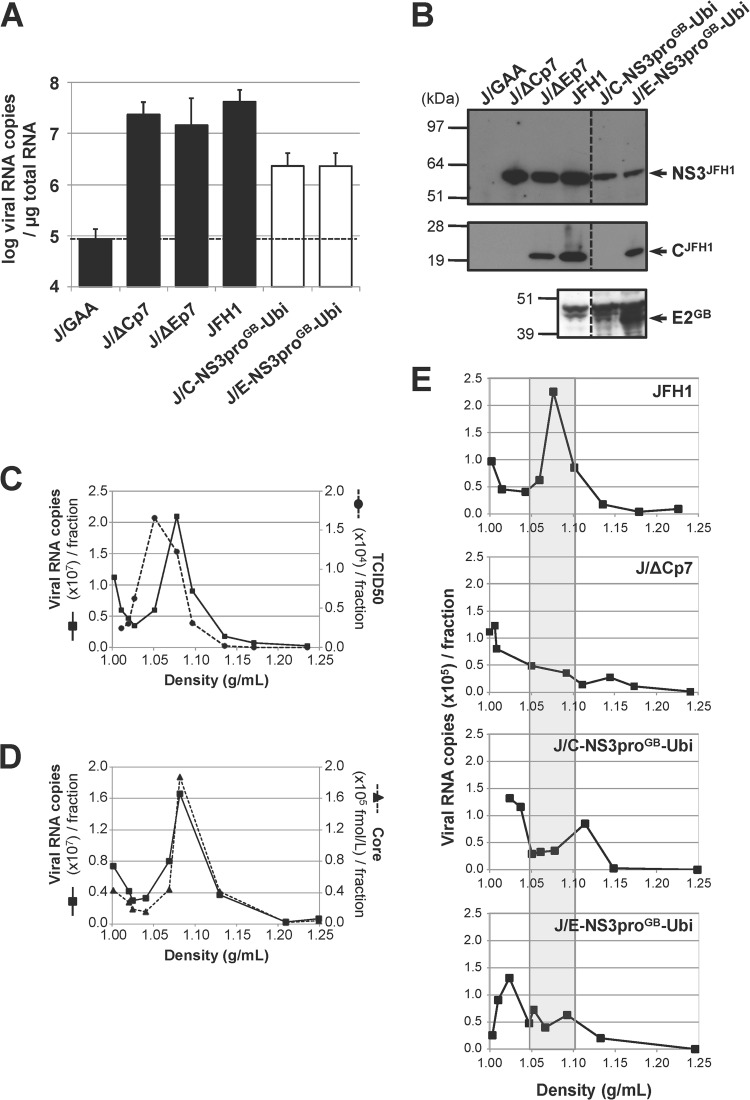
FIG 2.
Infectivity of chimeric GBV-B/JFH1 RNAs in Huh7.5 cells. (A) Replication activities of chimeric GBV-B/JFH1 RNAs. Huh7.5 cells were transfected with the parental JFH1 RNA, control RNAs (black bars), including the replication-deficient RNA carrying inactivating mutations in the polymerase active-site codons (J/GAA) and the assembly-deficient RNAs devoid of structural protein sequences (J/ΔCp7 and J/ΔEp7), or the indicated chimeric GBV-B/JFH1 RNAs (white bars). Viral RNA extracted from transfected cells at 96 h posttransfection was quantified by RT-qPCR relative to total cellular RNA normalized with respect to 18S RNA quantification. Mean results ± standard deviations from 3 independent transfections are shown. The dashed line indicates the threshold under which RNAs are nonreplicative. (B) Maturation of chimeric polyproteins. Protein extracts prepared from RNA-transfected cells at 96 h posttransfection were analyzed by SDS-PAGE followed by immunoblotting with anti-NS3JFH1, anti-CH77/JFH1, and anti-E2GB antibodies. (C to E) Viral particle production in transfected-cell supernatants. (C, D) The supernatant from JFH1 RNA-transfected Huh7.5 cells collected at 96 h posttransfection was concentrated over a 100-kDa exclusion membrane, loaded on a 10-to-40% iodixanol gradient, and ultracentrifuged to equilibrium. The RNA content (black lines, panels C and D), the infectivity (dashed line, panel C), and the core antigen content (dashed line, panel D) were determined in each of the 10 fractions collected, using RT-qPCR, TCID50 titration in Huh7.5 cells, and ELISA, respectively, and plotted as a function of density, measured by weighing each fraction. (E) Viral RNAs in the supernatants from cell cultures transfected with the indicated RNAs were collected at 96 h posttransfection, concentrated, and quantified by RT-qPCR. Adjusted volumes of concentrated supernatants corresponding to equal quantities of viral RNA were loaded on iodixanol gradients. Following ultracentrifugation, the RNA content of each fraction was determined by RT-qPCR. The shaded area delineates the density range at which RNA associated with JFH1 particles is expected to peak. The profiles shown are representative of 5 independent experiments.