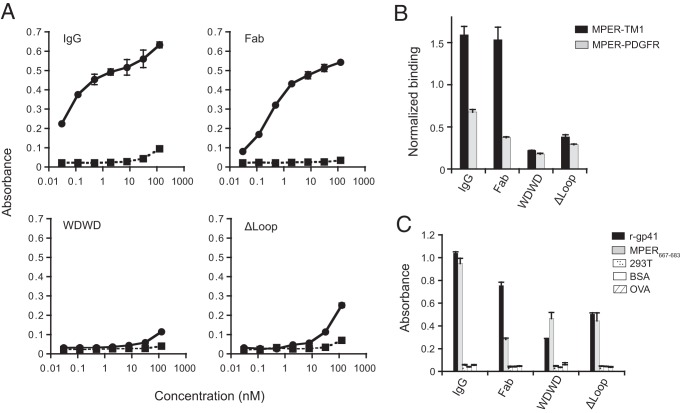
FIG 8.
Alterations in the CDR H3 of 4E10 Fab impacts the binding of Fab-s to its cognate epitope in the context of the plasma membrane. (A) Cell vesicles displaying the MPER-TM1 protein product (solid line), and “empty” control vesicles (dashed lines) were probed with 4E10 IgG and Fabs (WT, WDWD, and ΔLoop) at the indicated concentrations. (B) Cell vesicles bearing recombinant MPER proteins (MPER-TM1, black columns; MPER-PDGFR, gray columns) were probed with 20 nM 4E10 IgG and Fabs and 20 nM 17/9 IgG (which binds to an HA tag located at the N terminus of the MPER constructs and is used to assess cell surface expression). Absorbance values were normalized to the cell surface expression of the constructs (i.e., the absorbance of 4E10IgG or Fabs/the absorbance of 17/9IgG). (C) Binding of 20 nM 4E10 IgG and Fabs to recombinant gp41, the MPER667–683 peptide, and assay controls (“empty” 293T cell lysates, BSA, and ovalbumin). For all experiments, the absorbance at 405 to 490 nm was recorded. Error bars represent standard error of the mean. The data shown are from one of two (see Fig. 8B and C) or three (see Fig. 8A) experiments, all of which yielded similar results.