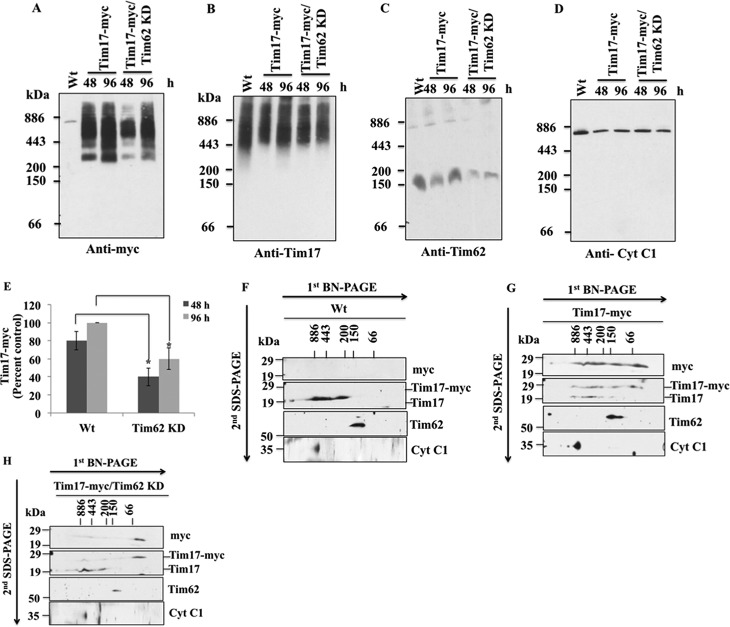
FIGURE 8.
Assembly of TbTim17 into the T. brucei mitochondrial membrane protein complexes. A–D, assembly of the ectopically expressed TbTim17–2X-myc in the mitochondrial protein complexes in T. brucei. Mitochondria were isolated from T. brucei Wt, which expressed TbTim17–2X-myc, and from T. brucei, which expressed this protein along with TbTim62 RNAi at days 2 and 4 after induction with doxycycline. Mitochondrial proteins were solubilized with 1% digitonin and analyzed by BN-PAGE. Proteins were transferred to nitrocellulose membrane and probed with anti-myc (A), anti-TbTim17 (B), anti-TbTim62 (C), and anti-Cyt c1 (D) antibodies. E, intensities of the Tim17-myc complex (∼300 to ∼1100 kDa) in Tim17-myc and Tim17-myc/Tim62 KD cells at 48 and 96 h after induction with doxycycline were quantitated and plotted as the percent of the maximum intensity of the complex found at 96 h in Wt background. S.E. are calculated from three independent experiments. *, p values <0.05. F–H, gel strips representing the individual lanes for Wt (F), TbTim17-myc (G), and TbTim17-myc/Tim62 KD (H) samples collected after 48 h of induction with doxycycline were excised from the first-dimension BN-PAGE gel and subjected to 12% Tricine SDS-PAGE. Proteins were transferred to a nitrocellulose membrane, and blots were sequentially probed with anti-myc, anti-TbTim17, anti-TbTim62, and anti Cyt c1 antibodies.