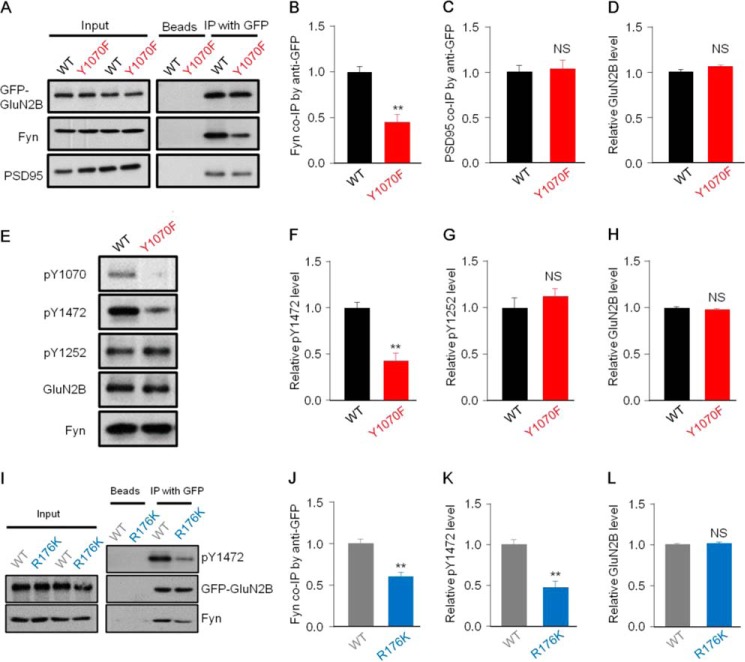
FIGURE 4.
Tyr-1070 is a critical site for GluN2B binding to Fyn and regulating the phosphorylation level of GluN2B at Tyr-1472. A–F, HEK293 cells were co-transfected with GluN1/GFP-GluN2BWT/Fyn or GluN1/GFP-GluN2BY1070F/Fyn. A, representative co-IP analysis with anti-GFP antibodies showing that remarkably decreased co-IP of Fyn with GluN2B in transfected cells co-expressing the GluN2BY1070F mutant compared with those co-expressing GluN2BWT, but the co-IP of PSD95 and GluN2B exhibited no difference between the two groups. B–D, statistical analysis of data from A (n = 7, **, p ≤ 0.01, two-tailed t test). NS, not significant. E, representative Western blots of co-transfected cells showing that the phosphorylation level of GluN2B at Tyr-1472, but not at Tyr-1252, was significantly reduced in transfected cells co-expressing the GluN2BY1070F mutant. F–H, quantitative analysis of Tyr(P)-1472, Tyr(P)-1252, and GluN2B levels in GFP-GluN2BWT- and GFP-GluN2BY1070F-transfected HEK293 cells (n = 4;, **, p ≤ 0.01, two-tailed t test). I, HEK293 cells were co-transfected with GluN1/GFP-GluN2B/FynWT or GluN1/GFP-GluN2B/FynR176K, and lysates were subjected to co-IP and immunoblotting. Immunoblotting revealed that the FynR176K mutant associated less with GluN2B, and the phosphorylation level of GluN2B Tyr-1472 was lower in transfected cells co-expressing FynR176K than in those co-expressing FynWT. J–L, statistical analysis for data as in F (n = 9; **, p ≤ 0.01, two-tailed t test).